Re: Why would you NOT take meds?
Posted: Wed Jul 02, 2008 2:01 am
Well, although they're not an ideal treatment, they're not entirely worthless.gwa wrote:I would not waste my time with any of the CRABS.
This paper demonstrated a reduction in brain atrophy in Avonex treated patients as well as an incyrease in the gray matter brain fraction.
Interferon beta-1a slows progression of brain atrophy in relapsing-remitting multiple sclerosis predominantly by reducing gray matter atrophy. Mult Scler. 2007 May;13(4):490-501.
BACKGROUND: Brain atrophy, as assessed by magnetic resonance imaging (MRI), has been correlated with disability in patients with multiple sclerosis (MS). Recent evidence indicates that both white matter (WM) and gray matter (GM) are subject to atrophy in patients with MS. Although neurological deficiencies in MS are primarily due to loss of WM, the clinical significance of GM atrophy has not been fully explored in MS. METHODS: We have undertaken a three-year, open-label study, comparing 26 patients who elected to receive intramuscular interferon beta-1a (IFN beta-1a) therapy, with 28 patients who elected not to receive therapy. Both groups had quantitative cranial MRI scans at study entry and after three years, and standardized clinical assessments every six months. Brain parenchymal fraction (BPF), GM fraction (GMF), and WM fraction (WMF) percent changes were calculated, and T2- and T1-lesion volumes (LVs) assessed. RESULTS: After three years, mean percent (%) change in BPF favored the IFN beta-1a treatment group (IFN beta-1a -1.3% versus the control group -2.5%, P=0.009), as did the mean percent change in GMF (+0.2 versus -1.4%, P=0.014), and the mean percent change in T1-LV (-9.3 versus +91.6%, P=0.011). At the end of the study, there was a significant within-patient decrease in BPF for both groups (P=0.02 for the IFN beta-1a treatment group, and P<0.001 for the control group), a significant within-patient decrease in WMF for the IFN beta-1a treatment group (P=0.01), and a significant decrease in GMF for the control group (P=0.013) when compared with baseline. CONCLUSION: Over a three-year period, treatment with IFN beta-1a significantly slowed the progression of whole-brain and GM atrophy, and of T1-hypointense LV accumulation, when compared with the control group.
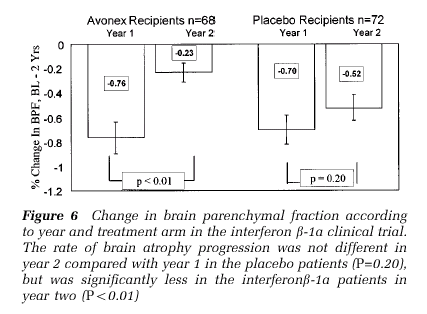
Moreover, this paper comparing Avonex to placebo showed that there was significantly less brain atrophy in the second year of Avonex use vs. placebo.
Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon beta-1a.
Mult Scler. 2000 Dec;6(6):365-72.
Brain atrophy is a relevant surrogate marker of the disease process in multiple sclerosis (MS) because it represents the net effect of various pathological processes leading to brain tissue loss. There are various approaches to quantifying central nervous system atrophy in MS. We have focused on a normalized measure of whole brain atrophy, the brain parenchymal fraction (BPF). BPF is defined as the brain parenchymal volume, divided by the volume within the surface of the brain. We applied this method to an MRI data set generated during a phase III clinical trial of interferon beta-1a (AVONEX). The purpose of the current study is to further explore clinical and MRI correlates of the BPF, particularly as they relate to relapse rate and Kurtzke's Expanded Disability Status Score (EDSS); and to further explore the therapeutic effects observed in interferon beta-1a recipients. Of all demographic and disease measures in the clinical trial data base, T2 lesion volume most closely correlated with BPF in cross sectional studies, and was the baseline factor most closely correlated with progressive brain atrophy in the subsequent 2 years. We also observed that change in T2 lesion volume was the disease measure most closely correlated with change in BPF during 2 years of observation. Of interest, relapse number and EDSS change during 2 years were only weakly correlated with BPF change during the same period. Disability progression, defined as sustained worsening of at least 1.0 EDSS points from baseline, persisting at least 6 months, was associated with significantly greater brain atrophy progression. We observed a therapeutic effect of interferon beta-1a in the second year of the clinical trial, and this beneficial effect was not accounted for by change in gadolinium enhanced lesion volume, or by corticosteroid medication within 40 days of the final MRI scan. The BPF is an informative surrogate marker for destructive pathological processes in relaping MS patients, and is useful in demonstrating treatment effects in controlled clinical trials. The significance of progressive brain atrophy during relapsing MS will be assessed by measuring clinical and MRI changes in prospective follow up studies.
Here's a data plot from the second paper...
NHE