javaneen wrote:Found this artical regarding high neurtralizing antibodies blocking the effect of interferons. My neuro tested me for this after 6 months of use of Betaseron when my 6 month MRI showed break through lesions. My blood test came back very high so he switched my medicine.
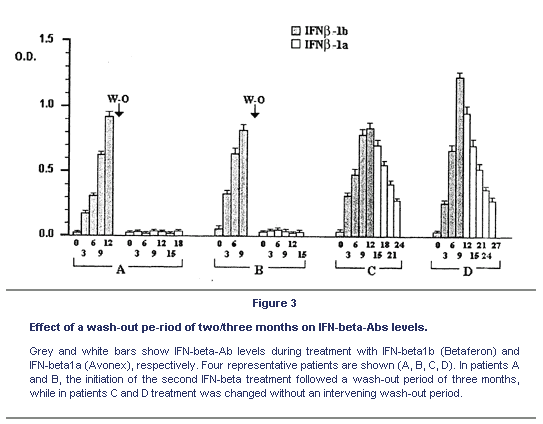
Several journal articles have reported that Betaseron (Ifn-B1b) is more antigenic than either Avonex or Rebif (Ifn-B1a). This is partly due to the fact that Ifn-B1b is produced in bacteria cells. Ifn-B is a normally glycosylated protein. This means that when our cells produce it they attached sugar groups to it. Bacteria do not glycosylate proteins. As a result, Ifn-B1b produced in bacteria will look more like a foreign protein to the immune system and will be more likely to elicit neutralizing antibodies (NAB). The journal article below shows that with patients on Ifn-B1b and with high levels of NAB, a short wash-out period eliminated the NAB and they did not return after switching over to Ifn-B1a.
I hope that this is helpful for you, NHE
Interferon-beta (INF-beta) antibodies in interferon-beta1a- and interferon-beta1b-treated multiple sclerosis patients. Prevalence, kinetics, cross-reactivity, and factors enhancing interferon-beta immunogenicity in vivo.
Eur Cytokine Netw. 2001 Mar;12(1):56-61.
We analysed the role of dosage, route and frequency of administration of clinical grade interferon-beta (IFN-beta) preparations in inducing anti-IFN-beta antibodies (IFN-beta-Abs) in 5 groups of relapsing-remitting multiple sclerosis (RRMS) patients who were respectively treated as follows: 1) weekly intramuscular (i.m.) injections of 30 mg of recombinant IFN-beta1a (Avonex), 2) subcutis (s.c.) injections of 250 mg IFN-beta1b (Betaferon) every other day, 3) weekly i.m. injections of 250 mg IFN-beta1b (Betaferon), 4) s.c. injections of 22 mg of IFN-beta1a (Rebif) three times a week, and 5) i.m. injections of 22 mg of IFN-beta1a (Rebif) twice a week. IFN-beta-Abs were determined by ELISA. IFN-beta1b was more immunogenic than IFN-beta1a not only when administered s.c. every other day, but also when administered i.m. at a lower weekly dose; i.m. injection, however, significantly delayed the appearance, and induced lower serum levels of IFN-beta-Abs. In patients treated with s.c. IFN-beta1b, Ab levels peaked 3 to 9 months after therapy initiation, and then slowly, but progressively, declined to pre-therapy levels that in some patients were reached after three years. Patients treated with i.m. or s.c. IFN-beta1a only rarely developed IFN-beta-Abs, and then at very low titers. Overall, the i.m. weekly administration of IFN-beta1a was the less immunogenic treatment. In IFN-beta1b-treated patients, a wash-out period of two/three months was sufficient to bring the IFN-beta-Ab levels below the cut-off. Our findings suggest that the immunogenicity of IFN-beta1a is low, regardless of the route of administration and the dosage, while that of IFN-beta1b is high, and is significantly, but not completely reduced by i.m. administration. As IFN-beta-Abs are cross-reactive, a wash-out period is suggested when the preparation is changed from IFN-beta1b to IFN-beta1a in order to maintain the clinical benefits of the therapy.