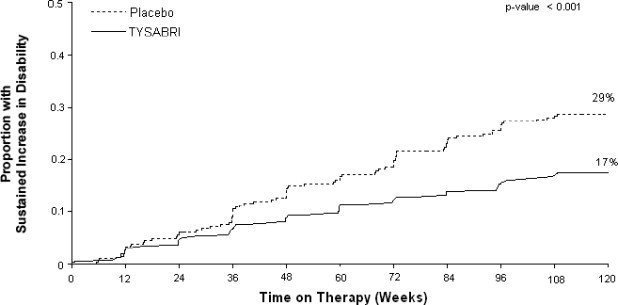
update on Tysabri--August 2013
Posted: Thu May 23, 2013 8:32 am
According to e-healthme, a reporting site which is based on reports by the FDA and self-reporting of actual patients, on May 22, 2013--
90,168 people reported to have side effects when taking Tysabri.
Among them, 665 people (0.74%) have died.
http://www.ehealthme.com/ds/tysabri/death
359 people are currently reported to have PML.
http://chefarztfrau.de/?page_id=716
But there have been more Tysabri deaths documented, linked to antibody reactions, lethal relapses after withdrawal, and cancer. These are not included in the PML numbers. We honestly do not know how many pwMS have died because of Tysabri.
Patients are now tested for the JC virus before beginning therapy. But many do not realize that just because you are JC- does not mean you will remain that way. It is possible to become JC+ after beginning treatment.
I've been reading about this online, as people who believed they were JC-, are now testing JC+ and are having to terminate Tysabri infusions. They are experiencing a rebound of the immune system and ending up worse off than they were before.
A lethal MS relapse after Tysabri withdrawal
http://www.ncbi.nlm.nih.gov/pubmed/23100404
more on the Tysabri rebound effect--
http://ms.about.com/od/treatments/a/The ... Effect.htm
Another problem is that PML looks like an MS relapse. It is very hard to tell the difference until a brain biopsy is taken after death. Because PML doesn't always show up in blood or CSF tests.
To those who have other options, please talk to your doctors before beginning this drug.
I put together this info for my husband, when his neuro was pushing Tysabri--even though he was doing well, with no inflammation, relapses or MS progression.
Many doctors use scare tactics and make promises of restored health to get patients to sign up for these infusions.
But there is no research showing that MS progression is altered by Tysabri.
please be well,
cheer
90,168 people reported to have side effects when taking Tysabri.
Among them, 665 people (0.74%) have died.
http://www.ehealthme.com/ds/tysabri/death
359 people are currently reported to have PML.
http://chefarztfrau.de/?page_id=716
But there have been more Tysabri deaths documented, linked to antibody reactions, lethal relapses after withdrawal, and cancer. These are not included in the PML numbers. We honestly do not know how many pwMS have died because of Tysabri.
Patients are now tested for the JC virus before beginning therapy. But many do not realize that just because you are JC- does not mean you will remain that way. It is possible to become JC+ after beginning treatment.
http://www.medpagetoday.com/clinical-co ... osis/32743Illustrating the need for follow-up testing, Bloomgren and colleagues noted the case of one patient whose initial blood sample was negative for JC virus antibodies. Another sample taken 13 months later was positive and the patient developed PML a few weeks later.
I've been reading about this online, as people who believed they were JC-, are now testing JC+ and are having to terminate Tysabri infusions. They are experiencing a rebound of the immune system and ending up worse off than they were before.
A lethal MS relapse after Tysabri withdrawal
http://www.ncbi.nlm.nih.gov/pubmed/23100404
more on the Tysabri rebound effect--
http://ms.about.com/od/treatments/a/The ... Effect.htm
Another problem is that PML looks like an MS relapse. It is very hard to tell the difference until a brain biopsy is taken after death. Because PML doesn't always show up in blood or CSF tests.
http://www.ncbi.nlm.nih.gov/pubmed/23338729The patient developed subacute onset of bilateral blindness following his 44th dose of natalizumab (Tysabri). Ophthalmologic examination was normal, the brain magnetic resonance imaging was not suggestive of PML, and cerebrospinal fluid analysis did not reveal the presence of JCV DNA. The patient was subsequently treated for a presumed multiple sclerosis relapse with high-dose corticosteroids. Two weeks after his 45th dose of natalizumab, he developed hemiplegia that evolved into quadriparesis. Repeated magnetic resonance imaging and cerebrospinal fluid studies were diagnostic for PML. Postmortem histopathological analysis demonstrated PML-associated white matter and cortical demyelination.
http://www.ncbi.nlm.nih.gov/pubmed/23252596Diagnosis of PML can be confounded in patients with multiple sclerosis (MS) if new demyelinating lesions develop, and the sensitivity of existing diagnostic tests is less than ideal. In the case presented here, four samples of cerebrospinal fluid were negative for JC virus DNA by polymerase chain reaction, yet brain biopsy eventually proved positive by immunohistochemistry.
To those who have other options, please talk to your doctors before beginning this drug.
I put together this info for my husband, when his neuro was pushing Tysabri--even though he was doing well, with no inflammation, relapses or MS progression.
Many doctors use scare tactics and make promises of restored health to get patients to sign up for these infusions.
But there is no research showing that MS progression is altered by Tysabri.
please be well,
cheer