Page 2 of 3
Posted: Fri Oct 19, 2007 6:22 am
by HarryZ
Lyon wrote:HarryZ wrote:When I spoke to him a couple of years ago, he told me that they didn't see that much of an improvement with their patient group.
Hi Harry,
What do you think he meant by that? Not much of an improvement of symptoms or not much slowing down of progression?
Bob
Bob,
The initial conversation that I had with the neuro was during the trials when of course, being double blinded, nobody knew who was actually getting the drug. The comment I was given at the time was that some patients became worse, some remained the same and others were showing improvement. Unfortunately I can't elaborate on what "showing improvement" or "becoming worse" meant since he wasn't specific.
The second time that I spoke to the neuro was after their trial group had completed the study. Again, he wasn't detailed in his opinion of Tysabri at the time but said that the results he experienced were mixed. I said to him at the time that Biogen had made press releases indicating that Tysabri was far superior than anything out there at the time and that's when he made the comment that Biogen didn't exactly have the best reputation in the medical world for giving totally accurate data in their trials. He went on further to say that Tysabri looked like it would be good for mild case MS....not surprising in that only patients who were I believe 2.0 or lower on the EDSS scale participated in the trials.
So it looks like depending on who you talk to one gets different opinions on Tysabri. Ewizabeth's neuro seemed to experience far better results than what people got here. Kind of sounds like what happens when it comes to MS medications, doesn't it?
Harry
Posted: Fri Oct 19, 2007 6:52 am
by robbie
some patients became worse, some remained the same and others were showing improvement.
isn't this what happens with multiple sclerosis anyway without the drugs?
Posted: Fri Oct 19, 2007 12:12 pm
by Lyon
HarryZ wrote: Kind of sounds like what happens when it comes to MS medications, doesn't it?
All too true! It always boils down to having to believe something but not knowing who or what to believe.
After my wife was diagnosed and her neuro said "pick a treatment" it quickly became obvious that inflammation, lesions and progression of disability were things to be avoided. It also became obvious that, despite their differences, all the crabs are considered to be about 30% efficacious.
What isn't easily determined for a newbie is "what exactly does the 30% efficacy mean?" in regards to inflammation, lesion formation and disability progression.
If each of the crabmakers REALLY felt their product was superior to the others, they would be tripping over their feet in order to document what exactly their drug does better than the others but I don't see that happening.
Bob
Comparing Apples, Oranges & Pineapples
Posted: Fri Oct 19, 2007 2:30 pm
by wintergreen
EDSS and relapse data depend on patient-provided information (whether that be by oral interview or by observation), all complicated by the "good day, bad day" [i.e. snapshot in time] factor.
Some MS treatments went thru FDA approval trials pre-revision of the EDSS scale, used different inclusion and exlusion criteria than others and used different criteria to define 'relapse'. That makes comparison between and among treatments from different trials difficult.
Perhaps the T1/T2 lesion MRI scan info is the best that can be relied on, at for purposes of making efficacy comparisons.
Other stuff FYI:
1.
http://appneurology.com/current_issue/
Applied Neurology - January 2006 Volume 2, Number 1
NEUROLOGY NEWS FEATURES: What's New in the Revised MS Diagnostic Criteria
2.
http://appneurology.com/showArticle.jht ... =201200724
Trying Times: Factoring Risk-Benefit Equations Into MS Therapeutics
July 01, 2007 Vol. 3 No. 7
3.
http://brain.hastypastry.net/forums/showthread.php?t=92
Comparing Apples, Oranges & Pineapples
Posted: Fri Oct 19, 2007 2:42 pm
by Lyon
Thanks for the info wintergreen, very informative!
Bob
Posted: Fri Oct 19, 2007 4:14 pm
by HarryZ
robbie wrote:some patients became worse, some remained the same and others were showing improvement.
isn't this what happens with multiple sclerosis anyway without the drugs?
Yep! My wife went 20 years, virtually symptom free after her first attack...and never took a thing for her MS...not that there was anything to take back in the 70's and 80's.
Harry
Posted: Fri Oct 19, 2007 4:19 pm
by HarryZ
If each of the crabmakers REALLY felt their product was superior to the others, they would be tripping over their feet in order to document what exactly their drug does better than the others but I don't see that happening.
Bob
And it won't happen because they aren't even sure just how their drugs work on MS patients!! What you see happening now is head to head trials with the competition in the hope that they win in a statistical contest. Then they claim superiority and gain that all important percentage or two share of the MS medication market....and that means millions of $$$$.
Harry
Posted: Fri Oct 19, 2007 8:08 pm
by LisaBee
Well, I am hoping, and have posted hopes elsewhere that the crabmakers (Lyon, I like that one) will focus more on MS subset response.
If we add a t to the end of crab we get CRABT, which doesn't sound so promising so I won't do that. We need another vowel, y'all.
I remember my neuro saying in 2004 that I was a good candidate for Tysabri - that was the original premise, that Tysabri was going to be the new drug for the newly diagnosed/mild cases. The impression I got from my neuro was similar to HarryZ's. Then came the PML, then the black box warning, and now a lot of neuros (with a few exceptions) in keeping with the FDA don't consider Tysabri as a first line treatment, but one to try for people who didn't do well on a CRAB. That is a major shift in treatment approach between the clinical trials and postmarket and it will be a challenge to interpret postmarket alongside the clinical trial followups because patient selection criteria is different.
Going back the original post, it seems that in this particular study, the clinical response in terms of EDSS was either/or, there was not a gradient, but rather any sign of uptick, yes/no. It might be too harsh to say a percentage weren't helped by Tysabi based on any movement in EDSS. Those people might have been helped, but just incompletely, Tysabri may have slowed the progression, but not halted it. The data, as presented in the summary, aren't sufficient to say.
I am glad, ewizabeth, that you are feeling better with it. That is a good outcome.
Posted: Sat Oct 20, 2007 12:31 pm
by Lyon
.
Re: Comparing Apples, Oranges & Pineapples
Posted: Sat Oct 20, 2007 2:41 pm
by NHE
Lyon wrote:If each of the crabmakers REALLY felt their product was superior to the others, they would be tripping over their feet in order to document what exactly their drug does better than the others but I don't see that happening.
Actually, Biogen does go out of their way to promote Avonex as "the only injectable therapy that is proven by the FDA to reduce relapses and slow the progression of disability."
Here's a comparison chart from their marketing materials.
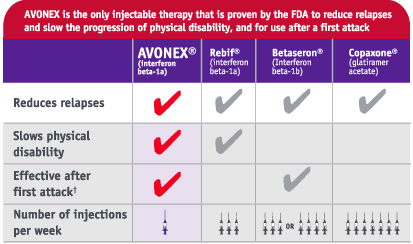
However, if you look at their two year data plots from their
Dr's Prescribing Information document, it really doesn't seem all that impressive.
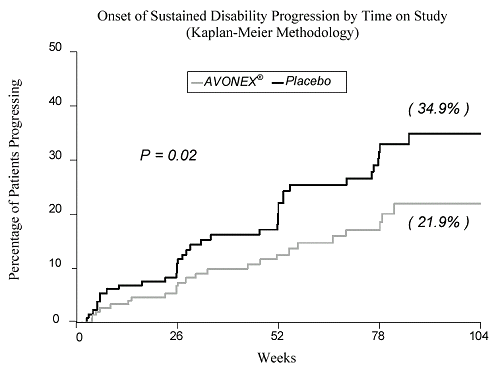
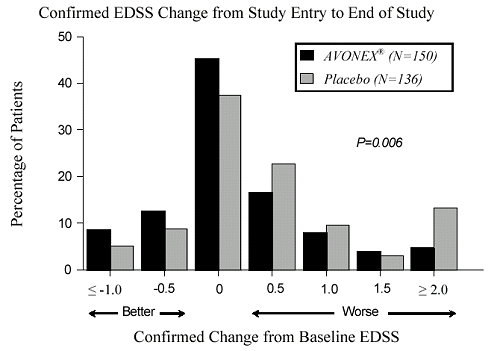
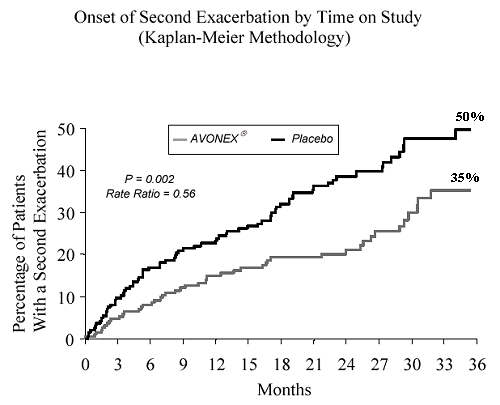
NHE
.
Posted: Sat Oct 20, 2007 3:10 pm
by Lyon
.
Re: Comparing Apples, Oranges & Pineapples
Posted: Sat Oct 20, 2007 4:22 pm
by HarryZ
Here's a comparison chart from their marketing materials.
I've always said that Biogen has the slickest marketing department of them all. Looking at that chart, it's almost a shoe-in that a patient would choose Avonex. Of course, in my opinion, it's a bunch of crap and I'm sure the other 3 competitors would have a lot to say about the chart as well.
Harry
Re: Comparing Apples, Oranges & Pineapples
Posted: Sun Oct 21, 2007 3:26 am
by NHE
Even in spite of the moderate benefit seen in the data plots I posted, I still feel that there are some advantages to Avonex over the other interferons. First, it's an intramuscular injection so there are no injection site reactions as seen with subcutaneous injections which the literature for Betaseron describes as injection site tissue necrosis. Moreover, as an intramuscular injection, the absorption efficiency of the drug is about 80% compared to about 40% for a subcutaneous injection (information which will need to be taken into account in order to make a comparison of the dosing of the different interferons). My last point is that unlike Betaseron, Avonex is raised in mammalian cells so the protein is in it's normally glycosylated state. This helps to lower the antigenicity of the medication thereby diminishing the appearance of neutralizing antibodies. Even with these benefits, having been on Avonex for 7 years, I'm starting to wonder if the modest benefit seen in the above data plots is worth it. It certainly wouldn't be if I had to pay the full retail cost for it but fortunately right now I don't. I also had to put up with pretty bad side effects for about 1½ years. However, one of the things that keeps me going on Avonex is that it reduces the number of enhancing lesions seen on MRI by about 50-60%. I know there is some argument over what role lesions play in disability progression. However, I think it's reasonable to go with the hypothesis that they represent some component of disease progression. Thus, reducing their appearance should be of benefit. Moreover, given the modest benefit seen in the data, I feel it's important to not rely upon any of the CRABs as the sole source of disease modifying treatment as there are many other things that we can do to slow the progression of the disease, e.g., omega-3 fish oils, curcumin, EGCG, lipoic acid, dietary changes, etc. Lastly, I must apologize since I realize that this was a thread about Tysabri and I did not mean to hijack it over to one about Avonex. We started discussing Biogen's products in general and that led to the discussion of Avonex.
NHE
Posted: Sun Oct 21, 2007 7:58 am
by itsjustme
Back to Tysabri...
I've come to believe that I should have been born in Missouri (not Chicago) as Missouri is the "Show me" state. In other words, I'll belive it when I see it and not a moment before then.
The last time I saw my neorologist was a few months ago and I wanted to post here about how he said, upon completion of my second infusion, that one month is still too early to expect to see any benefit; that I need to give it more time.
As I am sitting in my wheelchair, he went on to tell me about how he has other patients who came into his office using a cane, but now they don't need to use a cane. This he said in a matter-of-fact tone. Which made me wonder "How could you make such a dramatic statement and yet say it so casually?!". Maybe he was just being nice and also optimistic.
Should I belive this MS neurologist?
Whilst reading about some patents/discoveries on The Mylein Repair Foundation website I found this doctor's name listed as a co-author of a paper. So...he should know something or two...right? But yet I don't know.
"Show Me"
Posted: Sun Oct 21, 2007 8:32 am
by HarryZ
Showme,
Should I belive this MS neurologist?
Whilst reading about some patents/discoveries on The Mylein Repair Foundation website I found this doctor's name listed as a co-author of a paper. So...he should know something or two...right? But yet I don't know.
"Show Me"
I've been following MS research for over 40 years. I can't count the number of stories that I have heard involving MS patients trying almost every kind of therapy ever developed and amazingly leaving their wheel-chairs, walkers and/or canes behind. It DOES happen and nobody can explain why.
The real result, however, will show over months and months of using a particular medication and when it comes to those for MS, the results can be all over the map. The bottom line....if it works for you then continue to take the particular therapy.
Harry



