Page 1 of 5
Injection site reaction to Tovaxin
Posted: Sat Dec 30, 2006 5:23 pm
by IHaveMS-com
Hi to all,
I have corrected a posting on my site
http://www.ihavems.com concerning my having an injection site reaction. There is a quick link at the top of the site to the information. I have read numerous posts about looking forward to seeing an injection site reaction, and this may act as a reverse placebo effect. If a patient does not see an injection site reaction, they may believe they are in the placebo group. I will paste in the correction below.
I have never had an injection site reaction and no one in my study has every had an injection site reaction.
From Loobie -- I had absolutely 0 shot site reaction (which I know probably means bupkus), but I just wanna' know!
My family has a personal relationship with the Zhang family. During some of the trips to Houston, we have lunch with them or visit them at their labs. Before I started the study, we had many discussions about the mechanics of the T-cell vaccine and the prior studies that the Zhangs had conducted. One comment was that about 20% of the patients in the 1990's Baylor study had an injection site reaction, so it was something we were looking for as the trial proceeded.
You will not find anything in the journal articles about injection site reactions, and if and when they occur in the current trials, they will be noted as an adverse event, even though there really is nothing adverse about the event.
An addition -- Never say never. I did know about Cohen's work. He is on Opexa's scientific advisory board. In all of Zhang's papers, there is no mention of injection site reactions. I should have made that point.
The main reason for that is not that there were none, but rather that Zhang didn't include them in the paper. He was not going for FDA approval and minor adverse events are not worth including in preliminary research.
Thanks to NHE and CureOrBust T Cell Vaccination in Multiple Sclerosis Relapsing-Remitting Nonresponders Patients -
http://eng.sheba.co.il/img/upload/6/600_362.pdf
Best regards, Tim
If you are going into the extension study, you should read my post
http://www.thisisms.com/ftopict-4868.html There is a buildup period where each successive dose of vaccine stimulates the body to produce more T-cells to eliminate the MRTCs. Each injection also stimulates the immune system to produce memory WBC that continue producing the T-cells that remove the MRTCs as the body mistakenly produces them. You will start building protection with the first injection, but it may take 3 injections to get your immune system up to a level that can handle any large output of MRTCs, an attack.
This is from my website explaining the injection site reaction that I though I had.
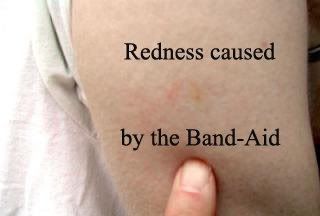
March 31, 2005 Three days after Tim received his injection, he removed the little round Band-Aid and discovered that there was redness at the injection site. Dr. Zhang had told Tim that about 20% of the people that he treated in the late 1990s had a reaction at the injection site. The redness was possibly a sign that the army of antibodies were attacking the attenuated myelin reactive T-cells from the vaccine and causing the redness at the point of injection. We were all delighted to see this. It would be 6 months before Tim would receive another injection, and at that time Dr. Loftus realized that the redness was from the Band-Aid and not from any battle between the protective T-cells generated by the vaccine and the injection of attenuated myelin reactive T-cells. Since plenty of time had passed since the original post, it didn't occur to us that we should go back and correct this erroneous observation. Tim has never had any redness at the injection site caused by a reaction to the vaccine. Tim has shown us some websites where people who are getting into the current study are hoping to see redness at the injection site, and he is concerned that if someone in the study does not see redness at the injection site, they will assume that they are in the placebo group. It should be obvious from Tim's experience that people in the study could see redness caused by a reaction to the Band-Aid or see redness caused by a reaction to the vaccine. The vast majority of patients should not see anything. If you know someone who is in the current study, please point this out to him or her.
This is the remainder of the post from 3/31/05 and the picture of the Band-Aid reaction. Notice how it has the outline of a little round Band-Aid. Tim will have his blood checked 4 months from now to see what level of myelin-reactive T-cells is present. A person with MS will continue to produce these bad T-cells, but by evaluating their blood and giving them the appropriate vaccine booster, the amount of myelin-reactive T-cells will approach zero and the destruction of myelin will stop. By analyzing the patient's blood every 6 months, the scientist is also able to look for any epitope (we are not going to try and explain that) shifts in the bad T-cells. The person's vaccine is constantly monitored and adjusted to keep the disease from ever doing damage again. This is personalized medicine. AUTOLOGOUS (the donor and recipient are the same individual) treatments have very little safety concerns, have no rejection problems, and are patient specific. For common short-term diseases, the one size fits all types of treatments are effective, but for autoimmune diseases, various types of cancer, and a host of other diseases, making the treatment using the patient's own cells is a medical missile that is programmed to seek out and destroy the problem.
Posted: Sat Dec 30, 2006 6:36 pm
by Lyon
Thanks Tim, your input is always appreciated.
I'd already read your site mentioning not to expect site reactions either way, so I wouldn't have been expecting any.
It's off topic but that situation always brings to mind that when my daughter was a toddler we took her to the HMO for a doctor's appointment where they always give kids a paper sticker for being "good" (yeah, right!)
She chose to put the sticker on her cheek, where it stayed for two or three hours. When I finally attempted to pull the sticker off I found that it had become PART of her face. I couldn't get it off without peeling her skin off and went into a panic. Finally I regained enough sense of mind to have her hold a wet washcloth on it until we were able to get it off but even then there was a big round red mark for a couple of days.
No doubt, bandaids and such are capable of leaving red marks.
Bob
Posted: Mon Jan 01, 2007 7:59 am
by Loobie
Tim,
Thanks for the post. I was having a shit day the day my shots were given as I didn't sleep much the night before. Not anticipatory insomnia, just my normal screwy sleeping pattern. You had even told me not to look for that and I still couldn't get it out of my head because of the way my nurse was about it.
I did, however, have a hightened Creatine kinase level in my blood for the labs that precede the shots. They said this could be after a good workout. Now I work out, but I had not worked out the day before this particular injection. I remember that because I went home right after the shot and ran since I knew I didn't do anything the day before. My research coordinator indicated that could be possibly showing that I am getting the drug, but I don't think he knows if that would be the case or not.
Once again, thanks for posting that info. about the shot site reactions. I was being a big puss when I posted that.
Lew
Posted: Mon Jan 01, 2007 11:34 am
by Lyon
Hi Lew,
It's only human nature to try to determine if you're on the "real thing" or not. The people most likely to register for clinical trials are the inquisitive and the risk takers. Those also happen to be the people who are going to go great lengths to determine if they are really on the treatment or placebo.
Of course the people running trials would find the most virgin results from participants who have no curiousity about the disease process or the treatment itself but those aren't the type of people who apply for clinical trials so our curiousity is just something they'll have to factor in.
Worsening of the symptoms is a legitimate cause of concern so make of it what you will, I've seen strong evidence that temporary worsening of symptoms is not uncommon in effective treatments for autoimmune disease. Specific to MS, researchers at least have noticed it in Campath 1H clinical trials.
Bob
Posted: Mon Jan 01, 2007 2:49 pm
by ewizabeth
Hi Tim,
This is reassuring to hear. So we should not assume that we are on the placebo if we do not have a reaction. My screening day is January 26th. I was happy to hear that people are finding out what their status is after 10 working days.
Thanks for the information, I appreciate it.
Posted: Thu Feb 22, 2007 1:15 pm
by Lyon
ewizabeth wrote: So we should not assume that we are on the placebo if we do not have a reaction.
It's always seemed kind of odd to me that people would even be looking for a site reaction after dosing with Tovaxin/placebo when considering that Tovaxin is just an aspect of their own blood. Seems to me that if Tovaxin produced a site reaction, giving blood would also produce a site reaction.....
Bob
Re: Injection site reaction to Tovaxin
Posted: Thu Feb 22, 2007 9:17 pm
by NHE
IHaveMS-com wrote:You will not find anything in the journal articles about injection site reactions, and if and when they occur in the current trials, they will be noted as an adverse event, even though there really is nothing adverse about the event.
I'm not an expert on T cell therapies by any means. However, at least one journal article does discuss injection site reactions. From one of my
earlier posts...
Thanks to CureOrBust for posting this paper from 2004 of an earlier, small, uncontrolled, phase I trial of a T cell vaccine.
T Cell Vaccination in Multiple Sclerosis Relapsing-Remitting Nonresponders Patients -
http://eng.sheba.co.il/img/upload/6/600_362.pdf
It mentions that of the 20 people in the study, only 55% experienced an injection site reaction and that this reaction usually didn't occur until after the second or third vaccination. In addition, the study only used two proteins, MBP and MOG, as opposed to the three that Opexa are using which also includes PLP. One of their primary findings was that the vaccination decreased the relapse rate and stabilized the EDSS with a small percentage improving.
NHE
Re: Injection site reaction to Tovaxin
Posted: Fri Feb 23, 2007 6:55 am
by Lyon
Hi NHE,
Thanks for including the Sheba link again. I remember the thread it was originally in but for some reason I didn't click on it at that point. I'm off work today and am enjoying reading it (did I REALLY say that I
enjoy reading research papers?!)
I think the point that Tim was trying to make was that Tovaxin clinical trial participants trying to determine if they are on the "real thing" by sight reaction are misguided.
Anytime you stick a needles in the arms of a large number of people there are going to be reports of sight reactions, but if the substance and the carrier are non irritants, the sight reactions can only be attributed to the different sensitivities of the participants, differing styles of the people administering the shots and the intensity of the speech the participants receive in regards to reporting sight reactions.
The above situation offers future recipients NO meaningful information needed to determine whether or not they are on the real thing by site reaction alone and it seems that is the situation in the Tovaxin trial.
Bob
Posted: Fri Feb 23, 2007 8:14 am
by Loobie
This is a funny topic to me after my experience yesterday. I went to get my fourth dose the other day and it could not have been touched more by Murphy's law if someone attempted to screw it up.
The first thing that happened is I was supposed to get my dose on Wed. the 21st right after the labs and blood work (which is totally typical). I then showed up back at the neuro's office to get dosed (my neuro. has an infusion clinic for Tysabri and trials and stuff) along with my other trial participant who has received only one dose to date.
That's when the fun started. Bob, I can't imagine what this scenario would have done to you since you have a four hour drive to the trial site for your wife. Anyway, it was foggy as hell on Tuesday since it warmed up and we had about 2 feet of snow on the ground. Long story short, the plane with the drugs could not take off from Memphis since it wasn't cleared to land in Dayton. So we got sent away to return at 7:00pm when they figured all would have been worked out. Little did they know... So we get called back later in the day saying that after they got cleared to land in Dayton, they couldn't take off in Memphis fue to freezing rain. So we scheduled for 9:30am the next day.
We show up for that and get told they still don't have the drugs. The FedEx guy who had on his instructions to get a signature, just dropped the drugs off, and not at the right location. He couldn't remember where, so they made him go back to evey stop. Anyway, we got sent away AGAIN. Everyone's anxiety is pinging because the drugs would be expired by 9:00 that night. They finally got the drugs and I ended up getting shot at around 3:30 yesterday afternoon.
Now for the point of the story and why I'm replying to an injection reaction thread. The woman who was getting dosed with me and I had some time to talk and she told me about the horrible reaction she received from her first dose. She said the entire left side of her face swelled up, but only for a short while. When I mentioned that to our research nurse (the one who does the injecting and who is, consequently the only who knows who is getting what) he fell off his chair laughing.
The reason I bring this up is that I would imagine that the anecdotal recountings of people's reaction to a drug rests entirely on the drama queen level of the person involved when it is not objectively identified. He told me that she had no reaction at all just like me. I really don't think we should expect a reaction at all; placebo or drug, but you never know.
Posted: Fri Feb 23, 2007 9:00 am
by Lyon
Loobie wrote: The reason I bring this up is that I would imagine that the anecdotal recountings of people's reaction to a drug rests entirely on the drama queen level of the person involved when it is not objectively identified. He told me that she had no reaction at all just like me. I really don't think we should expect a reaction at all; placebo or drug, but you never know.
Hi Lew,
WHEW!! I read your account with quite a bit of aprehension. You're right, I would have been a wreck but I'm glad to hear it resulted in a story of "all is well that ends well"!
You are absolutely right in that in a lot of trials, maybe most trials, differing site reactions might be expected because the product or the carrier is sometimes irritating to the skin.
In regards to when my wife was on Rebif, despite the fact that Rebif is derived from a human protein, something about it or the carrier is irritating so in a clinical trial against placebo I could easily see someone being able to tell that they were on Rebif if the administrator was a little sloppy during the injection process and got it on the skin.
In regards to Tovaxin, I assume that the carrier is saline solution, both for the placebo and the product and there is no reason to think that the T cells are in any way an irritant so the only site reaction should be due to the actual puncture and shouldn't in any way differentiate between the product or placebo.
Now for the point of the story and why I'm replying to an injection reaction thread. The woman who was getting dosed with me and I had some time to talk and she told me about the horrible reaction she received from her first dose. She said the entire left side of her face swelled up, but only for a short while.
That kind of situation is both funny and sad at the same time. The "funny" part is that the lady might very well have been injected with nothing but straight saline solution. The "sad" part is that this highlights the desperation of being diagnosed with MS. People who aren't normally risk takers become so desperate that they will enter clinical trials without the slightest real understanding of what they might have agreed to. I think luck was on that lady's side when she stood in the line for the Tovaxin trial as opposed to the line for the "liquid drano vs placebo" trial

I guess what really makes me sad and sympathetic is that I have a sister who is a real "nervous nellie" and if she were diagnosed with MS I can easily see her being like that lady. Overlooking the necessity to educate herself but enrolling in desperation. Yeah, in that situation the trauma alone would cause my sister to imagine all sorts of terrible things.
Bob
Posted: Sat Feb 24, 2007 12:20 pm
by IHaveMS-com
Never say never. I did know about Cohen's work. He is on Opexa's scientific advisory board. In all of Zhang's papers, there is no mention of injection site reactions. I should have made that point.
The main reason for that is not that there were none, but rather that Zhang didn't include them in the paper. He was not going for FDA approval and minor adverse events are not worth including in preliminary research.
I believe I sent Bob some information on the progression of the T-cell vaccine and he has it posted on his web page -- Zhang's 1st study late 1990s 40%, Cohen's study early 2000s 60%, Tovaxin study 2005 92%. The 2005 data was based on 6 peptide sequences. The vaccine that people in the current study will receive is derived using about 100 peptide sequences. The increased number of peptide sequences helps insure that the vaccine contains "all" of the MRTCs. No one can say they have found all of them, but the higher percentage that is found, the less MRTCs that will be left to cause problems.
The Tovaxin protocol uses an assay to detect myelin reactive T cells (MRTCs) from approximately 100 different peptide fragments over 3 of the major myelin proteins to identify an individual’s MRTC set. The initial screening determines if an individual has circulating MRTC's that react with these myelin peptide fragments (called epitopes). A negative result means that MRTC's could not be detected by the assay. The inability of the test to detect MRTCs may mean that an individual does not have adequate levels of MRTCs to be detected, that interfering substances may have been administered to the individual (such as steroids local and systemic) thereby rendering the MRTCs incapable of being detected in the assay.
Both people with MS and healthy subjects make MRTC's. In people without MS the immune system realizes that those cells are "self reactive" and they are not allowed to expand. In people with MS at certain times and for as yet unknown reasons, these MRTC's are allowed to expand. This often results in a flare-up that may result in a relapse.
Posted: Sat Feb 24, 2007 2:33 pm
by Lyon
IHaveMS-com wrote:The 2005 data was based on 6 peptide sequences. The vaccine that people in the current study will receive is derived using about 100 peptide sequences. The increased number of peptide sequences helps insure that the vaccine contains "all" of the MRTCs. No one can say they have found all of them, but the higher percentage that is found, the less MRTCs that will be left to cause problems.
Thanks for the additional information Tim. We were glad to get my wife into the Tovaxin IIb trial considering the situation as it was, so any improvements they make to the process is that much better!
I don't know how often you come around this site, and maybe this isn't even a valid question. I've had a long term obsession with helminth immunomodulation and in that regard there have been multiple clinical trials along the way. My experience with clinical trials is pretty much isolated to those situations. At any rate, in those trials it wasn't unusual that someone would experience an increase of symptoms before they experienced a decrease.
I know it's only one experience, but my curiousity forces me to ask. In your case did you notice any increase in symptoms after treatment or did you experience only improvement?
Thanks,
Bob
Posted: Sat Feb 24, 2007 8:57 pm
by IHaveMS-com
Hi Bob,
In your case did you notice any increase in symptoms after treatment or did you experience only improvement?
I only noticed an increase in ability. That is, I starting regaining lost function in the first month. That fact has to be taken with the fact that I was 26 when I started in the first study. I want to always emphasis that the role of Tovaxin is to halt the progression of the disease. Any disease reversal is do to the fact that the body is no longer under attack and is able to start repairing itself.
As to your other question --
After the immune system fights a specific enemy or experiences a vaccine, the "memory" of that specific pathogen or vaccine is supposedly stored in the B "memory" cells. The Tovaxin process specifically involves only the myelin reactive T cells but as far as I'm aware Tovaxin has no effect on the B cells. Might that be part of the reason that people being treated with Tovaxin have to be retreated ongoingly?
There are various types of White Blood Cells (WBCs also called leukocytes) that normally appear in the blood. The primary ones are T-lymphocytes, B-lymphocytes, and Monocytes.
The T-cells that attack myelin, like those that keep you from getting the chicken pox a second time, come from memory WBCs. Each time you are exposed to chicken pox, the memory WBCs produce T-cells to eliminate the chicken pox virus. Without exposure to the chicken pox virus, your memory WBCs will slowing decrease in number until you no longer have any disease protection.
It take 72 years of non-exposure for all of the memory WBCs to leave your system. I assume that number came from a mathematical model based on growth and decay, which was used to extrapolate the value. The value may be a good question for Trivial Pursuit, but the importance of the value is in understanding that immunity does not last forever.
The body maintains an ample supply of memory WBCs to instantly go into action and prevent the recurrence of a disease that a person has formed an immunity to. With an external invader, the body has time to generate a sufficient number of T-cells to stop the virus before it has time to replicate a sufficient number to make the person sick.
In the case of MRTCs, a person with MS can generate millions or billions of MRTCs in a day from the internal memory WBCs that produce them. This burst of production overwhelms the memory WBCs that the body has formed to generate T-cells to counteract the MRTCs and the person has an attack. These memory WBCs would have been produced in response to an injection of Tovaxin.
Because of this rapid internal production of MRTCs, a person with MS will need a booster every X months to be able to keep the good memory WBCs at a sufficient level to take out any rapid increase in MRTCs. The number of months between boosters will be individually determined. Some people may need vaccine every month and some may only need to be monitored.
With all that said, you are correct, Tovaxin has no effect on the memory WBCs that produce the MRTCs. If they could be eliminated, the vaccine would be a cure. Over time, if the person is not exposed to whatever it is that triggered the MS to begin with, the bad memory WBCs will slowly disappear.
Posted: Sat Feb 24, 2007 9:20 pm
by Lyon
Thanks on both counts Tim!
I appreciate the answers and I think I will "copy and paste" some of these questions and answers to my "Tovaxin" webpage, for my own future reference if nothing else.
Bob
Posted: Thu Mar 08, 2007 5:36 pm
by Loobie
That's some good information Tim. I must tell you that the anxiety from being in this placebo controlled trial, for me, comes from the wondering. I'm sure that is true in most trials. After I read some of the stuff that you explain it makes me feel very fortunate to be in this trial. I really am not sure if I'm getting the drug. I know I was having a relapse when I started and I just wonder if I feel better in the way that you do when you get done going through one. My eye is not getting worse, so I really should not complain.
If I find out I'm on the placebo, I'll feel really good since I feel good enough to do some light running now. I feel like I'm falling forward very pronouncedly when I get done jogging about a mile and a half, but I also feel very good about the fact that I feel good enough to attempt to run in the first place. There's a ton of tingling and numbness when I'm doing it, but it subsides shortly after I'm done. I also have to pee all the time when I'm running. But like I said, I'm just glad to be exercising like that. Thanks for the info. Tim.
Lew

