Bloomberg wrote:The most commonly prescribed multiple sclerosis drugs, including those made by Biogen Idec Inc. (BIIB), Bayer AG (BAYN) and Merck KGaA, failed to slow disability progression in a long-term study that raises new questions on whether the treatments can achieve that goal.
Researchers compared historical outcomes for MS patients in British Columbia to assess the use of interferon beta drugs. The results, published online in the Journal of the American Medical Association, found the medicines didn’t delay progress of the patients’ disability.
“It dampens somewhat the enthusiasm for so-called first- line therapies,” said Ludwig Kappos of University Hospital in Basel, Switzerland, and author of an editorial that accompanied the study, in an e-mail.
MS is an autoimmune disease that affects about 2.1 million people worldwide and can lead to limb numbness, loss of vision and paralysis, according to the National Multiple Sclerosis Society. The most common form, relapsing-remitting, is characterized by sporadic flare-ups followed by periods of inactivity.
Avonex, made by Biogen, Bayer’s Betaseron and Merck KGaA (MRK)’s Rebif generated $6.6 billion in 2011 revenue, according to data compiled by Bloomberg. Called disease-modifying drugs, they have been shown to slow the frequency of relapses and reduce the development of brain lesions. Their ability to slow disability progression has been less clear, wrote the researchers, led by Afsaneh Shirani of the University of British Columbia, in the paper reported yesterday.
Evidence Needed
“A key feature of MS is clinical progression of the disease over time manifested by the accumulation of disability,” the researchers wrote. “There is a lack of well- controlled longitudinal studies investigating the effect of interferon beta on disability progression.”
The study used data from the British Columbia Multiple Sclerosis database from 1985 to 2008. It compared three groups: 868 patients with relapsing-remitting MS who were treated with interferon beta therapy from July 1995 to December 2004; 829 patients who met the criteria to receive interferon beta therapy in that time period yet were untreated with it; and 959 patients who met the same criteria before interferon beta therapies became available in 1995 in Canada.
The use of two control groups sought to eliminate the chance of bias based on patients choosing not to receive therapy for reasons such as less severe disease, the researchers wrote.
Disability Measurement
The analysis considered advancement to a score of 6 on the Expanded Disability Status Scale, or EDSS, a commonly used metric to measure disability progression in MS. That score means a patient requires help from a cane or crutch to walk about 100 meters.
The study found that 10.8 percent of patients in the treated group reached an EDSS score of 6, compared with 5.3 percent in the contemporary untreated group and 23.1 percent in the historical untreated group.
“We did not find evidence that administration of interferon beta was associated with a reduction in disability progression in patients with relapsing-remitting MS,” the researchers wrote. “Our findings bring into question the routine use of interferon beta drugs to achieve this goal in MS.”
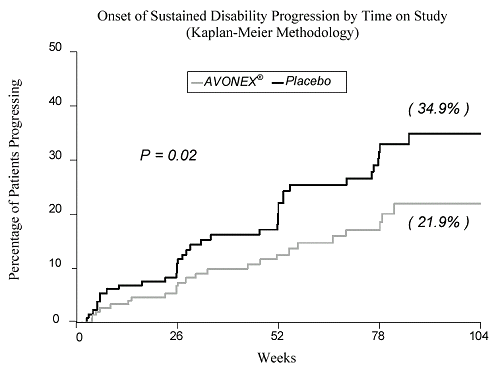
Biogen, based in Weston, Massachusetts, said its Avonex has been shown in clinical trials to slow disability progression.
‘Positive Impact’
“Regulators worldwide have reviewed and confirmed the positive impact of Avonex on slowing disease progression,” Jeff Boyle, a spokesman for the drugmaker, wrote in an e-mail. “In fact, Avonex is indicated for the treatment of patients with relapsing forms of multiple sclerosis to slow the accumulation of physical disability and decrease the frequency of clinical exacerbations.”
Merck, based in Darmstadt, Germany, said its Rebif has shown to be effective in “all three key measures of treatment efficacy: reducing relapses, delaying disability progression and reducing active brain lesions,” Heather Connor, a spokeswoman for the company’s EMD Serono unit, wrote in an e-mail. “The overall clinical benefits of disease-modifying therapies, including Rebif (interferon beta-1a) have been established in well-controlled Phase 3 clinical studies.”
The historical data used in the study doesn’t reflect current treatment practice, said Marcy Funk, a spokeswoman for Leverkusen, Germany-based Bayer.
Conversion Delay
“The results of the Phase 3 Benefit study showed that Betaseron treatment at the early stage of the disease can significantly delay conversion to clinically definite MS,” Funk wrote in an e-mail. Current practice, she said, “encourages treatment at the first sign of relapsing-remitting MS.”
The results don’t mean neurologists should immediately change prescribing practices, said Kappos, the editorial author.
“I don’t think it should change clinical practice as long as we do not have better options, but it reminds us of the need to better define a target population of responders to the established compounds and to find not only rather safe but more effective treatments,” he wrote.
Newer therapies such as Biogen’s Tysabri and Novartis AG (NOVN)’s Gilenya, the first oral therapy approved for MS, are examples of more effective treatments, Kappos said.
Added Risk
Kappos also cited their potential for increased risks. Tysabri, Biogen’s second best-selling medicine after Avonex, with $1.1 billion in 2011 revenue, is associated with a danger of contracting a brain infection called progressive multifocal leukoencephalopathy, or PML. The company has developed a test to help determine patients’ risk.
Teva Pharmaceutical Industries Ltd. (TEVA) sells Copaxone, another older MS therapy that isn’t in the same interferon beta class. While it wasn’t evaluated in this study, Copaxone doesn’t necessarily offer a better chance of delaying disability progression as it “has been shown to be very similar to the interferons in head-to-head trials,” Kappos wrote.
Teva declined to comment on the study, Denise Bradley, a company spokeswoman, said.
The data’s implications may be limited because the study was designed to show a 40 percent risk reduction with interferon treatment, more than the 30 percent shown in trials of the interferon beta therapies or Copaxone, Kappos and Tobias Derfuss, also of University Hospital in Basel, wrote in their editorial.
“Therefore, it is likely that neurologists will continue to prescribe interferon beta and other interferons and patients with relapsing-remitting MS will continue to self-inject these agents,” they wrote. “However, the rigorously collected data of Shirani and colleagues reinforce the conclusion that the associations between use of interferons and long-term disability, although plausible, remain unproven.”
To contact the reporter on this story: Meg Tirrell in New York at
mtirrell@bloomberg.net
To contact the editor responsible for this story: Reg Gale at
rgale5@bloomberg.net