What Is the Safest Cookware to Use?
What Is the Safest Cookware to Use?
What Is the Safest Cookware to Use?
A couple of years ago, I did research on this subject and ended up switching to cast iron. cookware Cast iron is still highly regarded for many reasons. But Gristy56's revelations regarding metals in the body, magnetic fields and his "Desatascador" (all of which seems make reasonable sense to me) and his theories behind it has gotten me to rethink my decision.
Although modern non-stick pans are thought to be relatively safe, I have always been and am still leery of them. The formulation in Teflon pans changed in 2013 to be PFOA free. Most modern non stick pans are PTFE-, PFOA-, and cadmium-free. 'Cheap' non stick pans should probably be avoided. Excellent ceramic nonstick coating are also available.
I have high end stainless steel pots and pans as well which I have previously felt comfortable with. Aluminum is a common material in a lot of current cookware but aluminium is thought to cause numerous health issues. A lot of pots and pans are made from aluminum and then covered with a nonstick material. Copper seems to be the rage now - I am sure you have seen the copper non-stick pans which are actually aluminum pans covered with a copper based non-stick coating. Ultimately every site I visit when researching materials used for pots has issues with just about all of them.
As far as non-stick goes, there are currently many different approaches to non-stick surfaces and all seem to work well and are probably safe to use. But I still choose to avoid them.
I tend to do a lot of my cooking on a grill, my latest one, a small pellet grill, has a ceramic coated cast iron grate. I am reasonably comfortable with that. (If you like to BBQ, when it comes to flavor, there is nothing like a pellet grill although smoking foods poses its own issues - I do use it at higher temperatures which minimizes the amount of smoke greatly.)
I also have an electric grill as well that came with a nonstick grate. I converted that to use a cast iron grate instead. But I think now I am going to get a ceramic coated cast iron grate instead.
But for general cooking, based on Gristy56's information about deposits of magnetites within the body, I have decided to give up all my cast iron cookware to pretty much the only material that does not typically leech metals and chemicals into the food and/or air - GLASS.
BUT, glass cookware is not perfect by any means - it can still can have a number of safety risks particularly if it is older glass or if it's country of origin is not known.
What kind of cookware do you use? Do you have any concerns about it?
A couple of years ago, I did research on this subject and ended up switching to cast iron. cookware Cast iron is still highly regarded for many reasons. But Gristy56's revelations regarding metals in the body, magnetic fields and his "Desatascador" (all of which seems make reasonable sense to me) and his theories behind it has gotten me to rethink my decision.
Although modern non-stick pans are thought to be relatively safe, I have always been and am still leery of them. The formulation in Teflon pans changed in 2013 to be PFOA free. Most modern non stick pans are PTFE-, PFOA-, and cadmium-free. 'Cheap' non stick pans should probably be avoided. Excellent ceramic nonstick coating are also available.
I have high end stainless steel pots and pans as well which I have previously felt comfortable with. Aluminum is a common material in a lot of current cookware but aluminium is thought to cause numerous health issues. A lot of pots and pans are made from aluminum and then covered with a nonstick material. Copper seems to be the rage now - I am sure you have seen the copper non-stick pans which are actually aluminum pans covered with a copper based non-stick coating. Ultimately every site I visit when researching materials used for pots has issues with just about all of them.
As far as non-stick goes, there are currently many different approaches to non-stick surfaces and all seem to work well and are probably safe to use. But I still choose to avoid them.
I tend to do a lot of my cooking on a grill, my latest one, a small pellet grill, has a ceramic coated cast iron grate. I am reasonably comfortable with that. (If you like to BBQ, when it comes to flavor, there is nothing like a pellet grill although smoking foods poses its own issues - I do use it at higher temperatures which minimizes the amount of smoke greatly.)
I also have an electric grill as well that came with a nonstick grate. I converted that to use a cast iron grate instead. But I think now I am going to get a ceramic coated cast iron grate instead.
But for general cooking, based on Gristy56's information about deposits of magnetites within the body, I have decided to give up all my cast iron cookware to pretty much the only material that does not typically leech metals and chemicals into the food and/or air - GLASS.
BUT, glass cookware is not perfect by any means - it can still can have a number of safety risks particularly if it is older glass or if it's country of origin is not known.
What kind of cookware do you use? Do you have any concerns about it?
Last edited by ElliotB on Fri Aug 10, 2018 5:06 am, edited 4 times in total.
Re: What Is the Safest Cookware to Use?
2018 Jun 6
Faculty of Health Sciences, Hacettepe University, Ankara, Turkey
Is aluminum exposure a risk factor for neurological disorders?
full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6040147/
Abstract
Aluminum (Al) is widely found in the nature. Although the relation between Al and neurodegenerative diseases is still controversial, Al is related with many brain diseases including Alzheimer's disease, Parkinson's disease, and multiple sclerosis. Al exposure occurs mainly through environment, occupational, and dietary factors for humans. Al exposure with diet can be through foods, food additives, water, and contamination of Al equipment/utensils. The aim of this review is to summarize various hypotheses, which link Al and neurodegeneration, and to determine the roles of Al exposure through different sources including diet, environment, and occupation. Future studies should be done in vulnerable subgroups of population including children, patients receiving antacid or Al-containing pharmeteucials on a daily basis, patients with reduced renal function, and patients on parenteral nutrition regimens that are likely to be affected by possible adverse health effects of Al. In addition, gender, age, and Al interactions need to be determined. One of the most important challanges in future epidemiological studies is to determine which variables should be controlled. In addition, experimental studies should be more focused and translational. In this context, exposure dose, dose-response effects, and time lapse between exposures and cognitive assessments are very important.

Faculty of Health Sciences, Hacettepe University, Ankara, Turkey
Is aluminum exposure a risk factor for neurological disorders?
full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6040147/
Abstract
Aluminum (Al) is widely found in the nature. Although the relation between Al and neurodegenerative diseases is still controversial, Al is related with many brain diseases including Alzheimer's disease, Parkinson's disease, and multiple sclerosis. Al exposure occurs mainly through environment, occupational, and dietary factors for humans. Al exposure with diet can be through foods, food additives, water, and contamination of Al equipment/utensils. The aim of this review is to summarize various hypotheses, which link Al and neurodegeneration, and to determine the roles of Al exposure through different sources including diet, environment, and occupation. Future studies should be done in vulnerable subgroups of population including children, patients receiving antacid or Al-containing pharmeteucials on a daily basis, patients with reduced renal function, and patients on parenteral nutrition regimens that are likely to be affected by possible adverse health effects of Al. In addition, gender, age, and Al interactions need to be determined. One of the most important challanges in future epidemiological studies is to determine which variables should be controlled. In addition, experimental studies should be more focused and translational. In this context, exposure dose, dose-response effects, and time lapse between exposures and cognitive assessments are very important.

https://www.eboro.cz
Re: What Is the Safest Cookware to Use?
Zinc Supplementation Alters Plasma Aluminum and Selenium Status of Patients Undergoing Dialysis: A Pilot Study
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3705357/
"Zn supplementation ameliorates abnormally high plasma Al concentrations and oxidative stress and improves Se status in long-term dialysis patients."
Zinc Deficiency-induced Iron Accumulation, a Consequence of Alterations in Iron Regulatory Protein-binding Activity, Iron Transporters, and Iron Storage Proteins
http://www.jbc.org/content/283/8/5168.short
"zinc deficiency can result in alterations in iron transporter, storage, and regulatory proteins, which facilitate iron accumulation"
Disorders of metal metabolism
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5764069/
"Transition metals are strictly defined as elements whose atom has an incomplete d sub-shell. This incomplete d sub-shell makes them prone to chemical reactions, particularly redox reactions. Transition metals of biologic importance include copper, iron, manganese, cobalt and molybdenum. Zinc is not a transition metal, since it has a complete d sub-shell."
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3705357/
"Zn supplementation ameliorates abnormally high plasma Al concentrations and oxidative stress and improves Se status in long-term dialysis patients."
Zinc Deficiency-induced Iron Accumulation, a Consequence of Alterations in Iron Regulatory Protein-binding Activity, Iron Transporters, and Iron Storage Proteins
http://www.jbc.org/content/283/8/5168.short
"zinc deficiency can result in alterations in iron transporter, storage, and regulatory proteins, which facilitate iron accumulation"
Disorders of metal metabolism
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5764069/
"Transition metals are strictly defined as elements whose atom has an incomplete d sub-shell. This incomplete d sub-shell makes them prone to chemical reactions, particularly redox reactions. Transition metals of biologic importance include copper, iron, manganese, cobalt and molybdenum. Zinc is not a transition metal, since it has a complete d sub-shell."
active members shape site content. if there is a problem, speak up!
use the report button to flag problematic post content to volunteer moderators' attention.
use the report button to flag problematic post content to volunteer moderators' attention.
Re: What Is the Safest Cookware to Use?
Zinc is in group IIB of the periodic table as are cadmium and mercury. While it is true that these atoms have a filled d electron orbital, they are still transition metals.jimmylegs wrote:Disorders of metal metabolism
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5764069/
"Transition metals are strictly defined as elements whose atom has an incomplete d sub-shell. This incomplete d sub-shell makes them prone to chemical reactions, particularly redox reactions. Transition metals of biologic importance include copper, iron, manganese, cobalt and molybdenum. Zinc is not a transition metal, since it has a complete d sub-shell."
Here is the text from my periodic table program describing transition metals.
Here's a useful site. https://ptable.com/ Note: Clicking on the Zn symbol brings up the Wikipedia page that calls Zn a "post transition" metal. However, post transition metals are those where the p orbitals are being filled.Transition Metal Elements
This group of metals are distinguished from other metals not by their physical properties, but by their electronic structure. The transition metals have their valence electrons in more than one shell. Regular, or representative, metals have their valence electrons in only one shell.
The transition metals include the elements of the groups IIIA through IIB in each of the long periods of the periodic table.
The transition metals are noted for their variability in oxidation state. This is attributed to the presence of valence electrons in more than one shell. Whereas, the representative metals have only one or two oxidation states, since they have valence electrons in only one shell.
Thus, manganese has two electrons in its outside shell and five electrons in the next shell down, and exhibits oxidation states of +1, +2, +3, +4, +5, +6, and +7.
They are also characterized by the fact that well into the series, going from left to right, the properties of succeeding metals do not differ greatly from preceding ones. This is attributed to the fact that, generally, succeeding elements differ in electronic structure by one electron in the next to the outer valence shell rather than the outer shell.
In contrast, those of succeeding representative metals in a period differ extensively, since they differ by one electron in the outer valence shell.
Re: What Is the Safest Cookware to Use?
related
https://www.ajol.info/index.php/njps/article/view/95096
"Zinc is classified as a group 11B post-transition metal. In biological systems, it exists as Zn2+ and is present in all tissues and fluids in the body (European
Commission,2003)"
Trace elements in human physiology and pathology: zinc and metallothioneins
https://www.sciencedirect.com/science/a ... 2203000817
"The zinc ion (Zn++) does not participate in redox reactions, which makes it a stable ion in a biological medium whose potential is in constant flux."
https://en.wikipedia.org/wiki/Transition_metal
"Zinc, cadmium, and mercury are generally excluded from the transition metals[5] as they have the electronic configuration [ ]d10s2, with no incomplete d shell.[13] In the oxidation state +2 the ions have the electronic configuration [ ] d10.
However, these elements can exist in other oxidation states, including the +1 oxidation state, as in the diatomic ion Hg2+2. The group 12 elements Zn, Cd and Hg may therefore, under certain criteria, be classed as post-transition metals in this case."
https://www.ajol.info/index.php/njps/article/view/95096
"Zinc is classified as a group 11B post-transition metal. In biological systems, it exists as Zn2+ and is present in all tissues and fluids in the body (European
Commission,2003)"
Trace elements in human physiology and pathology: zinc and metallothioneins
https://www.sciencedirect.com/science/a ... 2203000817
"The zinc ion (Zn++) does not participate in redox reactions, which makes it a stable ion in a biological medium whose potential is in constant flux."
https://en.wikipedia.org/wiki/Transition_metal
"Zinc, cadmium, and mercury are generally excluded from the transition metals[5] as they have the electronic configuration [ ]d10s2, with no incomplete d shell.[13] In the oxidation state +2 the ions have the electronic configuration [ ] d10.
However, these elements can exist in other oxidation states, including the +1 oxidation state, as in the diatomic ion Hg2+2. The group 12 elements Zn, Cd and Hg may therefore, under certain criteria, be classed as post-transition metals in this case."
active members shape site content. if there is a problem, speak up!
use the report button to flag problematic post content to volunteer moderators' attention.
use the report button to flag problematic post content to volunteer moderators' attention.
Re: What Is the Safest Cookware to Use?
Zn2+ is not magic. It can be reduced. The standard reduction potential for Zn2+ is...jimmylegs wrote:Trace elements in human physiology and pathology: zinc and metallothioneins
https://www.sciencedirect.com/science/a ... 2203000817
"The zinc ion (Zn++) does not participate in redox reactions, which makes it a stable ion in a biological medium whose potential is in constant flux."
Zn2+(aq) + 2 e- ---> Zn(s), E° = -0.763 V.
Example: Zn2+(aq) + H2(g) ---> Zn(s) + 2H+(aq)
Put a copper plated zinc penny in some acidified hydrogen peroxide and you can watch the reaction go in reverse, i.e., Zn(s) ---> Zn2+(aq) + 2e-(aq). In fact, acidified hydrogen peroxide has a very strong reduction potential, E° = +1.78 V, and should easily dissolve (oxidize) many metals.
Re: What Is the Safest Cookware to Use?
funny how these papers are making it past peer review with such fundamental errors right up front.
re copper plated zinc penny in acidified hydrogen peroxide... the parallel biological / human physiological setting would be...??
and what are the implications of this scenario in humans, for zinc's handling of metals associated with cookware choices, like iron and aluminum ?
re copper plated zinc penny in acidified hydrogen peroxide... the parallel biological / human physiological setting would be...??
and what are the implications of this scenario in humans, for zinc's handling of metals associated with cookware choices, like iron and aluminum ?
active members shape site content. if there is a problem, speak up!
use the report button to flag problematic post content to volunteer moderators' attention.
use the report button to flag problematic post content to volunteer moderators' attention.
Re: What Is the Safest Cookware to Use?
Nothing of course. It's just fun to do a little chemistry at home. Oh, you can put a drop of bleach on a penny. Not much happens. However, add some grains of table salt and it will erode pits into it.jimmylegs wrote:re copper plated zinc penny in acidified hydrogen peroxide... the parallel biological / human physiological setting would be...??
Avoid aluminum. 25 years ago I used to use an aluminum pot for cooking rice. I switched to stainless steel.jimmylegs wrote:and what are the implications of this scenario in humans, for zinc's handling of metals associated with cookware choices, like iron and aluminum ?
Re: What Is the Safest Cookware to Use?
2018 Jul 26
Department of Neurology, Otto-von-Guericke-University, Magdeburg, Germany
Lower Serum Zinc Levels in Patients with Multiple Sclerosis Compared to Healthy Controls.
https://www.ncbi.nlm.nih.gov/pubmed/30049983
CONCLUSIONS:
The data suggest that a diagnosis of MS is related to lower serum zinc concentrations than in HCs, and concentrations were lower still under disease-modifying therapy. However, zinc levels did not predict disease subtypes or disability status.
full text: http://www.mdpi.com/2072-6643/10/8/967/htm
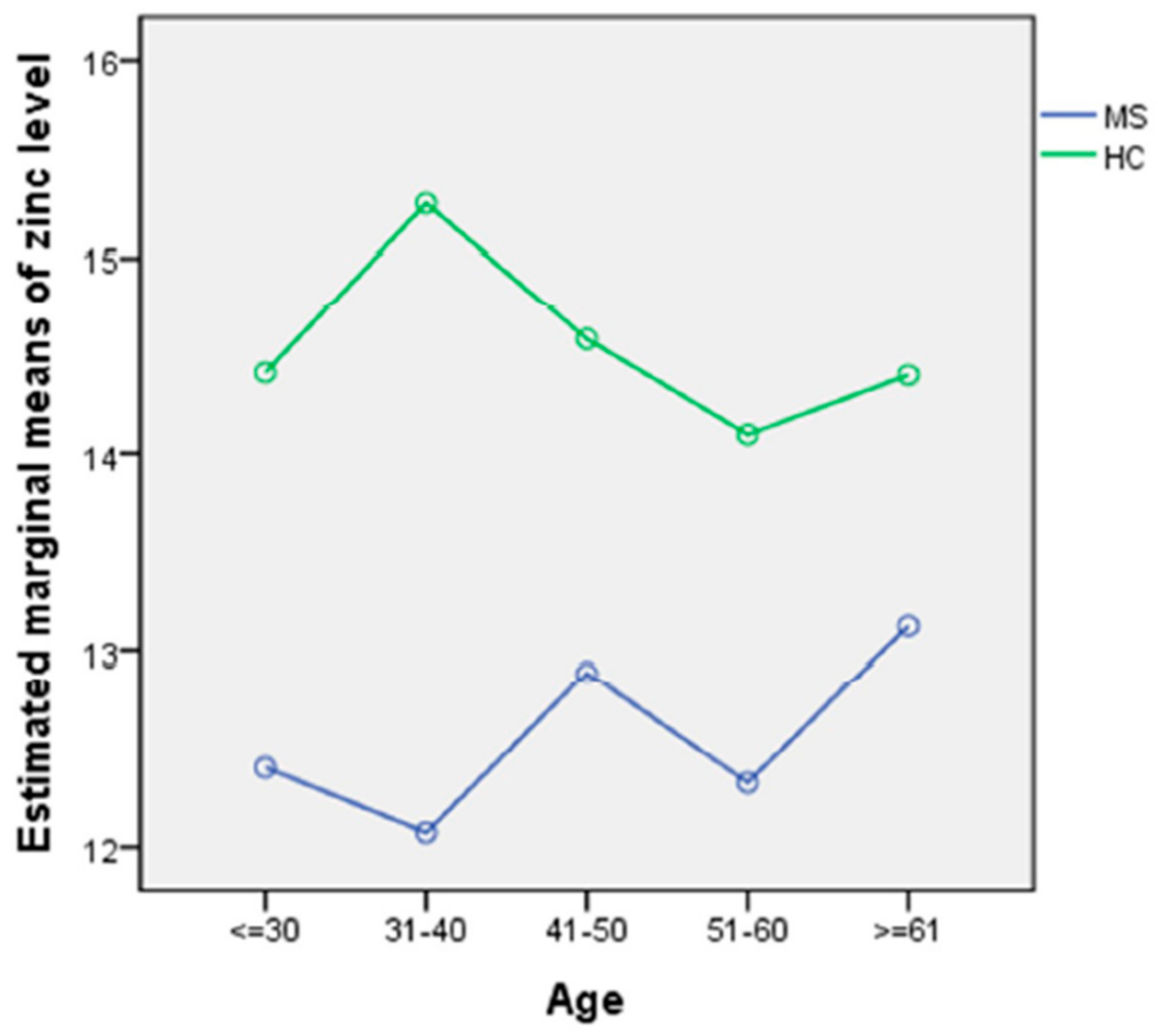
- healthy controls (HCs)
Department of Neurology, Otto-von-Guericke-University, Magdeburg, Germany
Lower Serum Zinc Levels in Patients with Multiple Sclerosis Compared to Healthy Controls.
https://www.ncbi.nlm.nih.gov/pubmed/30049983
CONCLUSIONS:
The data suggest that a diagnosis of MS is related to lower serum zinc concentrations than in HCs, and concentrations were lower still under disease-modifying therapy. However, zinc levels did not predict disease subtypes or disability status.
full text: http://www.mdpi.com/2072-6643/10/8/967/htm

- healthy controls (HCs)
https://www.eboro.cz
Re: What Is the Safest Cookware to Use?
re
nice recent research find petr. i have added it, with comment, to the catalog of zinc-related info posted under natural approach
my point above was, as always, about optimizing essentials before implementing crutches like avoidance into less than optimally functional systems:Avoid aluminum. 25 years ago I used to use an aluminum pot for cooking rice. I switched to stainless steel.
that said, the main pots in use here for preparing foods at a boil are stainless, and the main pans for frying are cast iron. i'm not shy about beverages canned in aluminum or aluminum foil - not to say that they form part of the every day routine, of course. my last zinc test was garbage - i put myself on a month long regimen of 90mg/d in order to get topped up.Zinc Supplementation Alters Plasma Aluminum and Selenium Status of Patients Undergoing Dialysis: A Pilot Study
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3705357/
"Zn supplementation ameliorates abnormally high plasma Al concentrations and oxidative stress and improves Se status in long-term dialysis patients."
nice recent research find petr. i have added it, with comment, to the catalog of zinc-related info posted under natural approach
active members shape site content. if there is a problem, speak up!
use the report button to flag problematic post content to volunteer moderators' attention.
use the report button to flag problematic post content to volunteer moderators' attention.
Re: What Is the Safest Cookware to Use?
How is avoiding aluminum cookware a "crutch?"jimmylegs wrote:my point above was, as always, about optimizing essentials before implementing crutches like avoidance into less than optimally functional systemsNHE wrote:Avoid aluminum. 25 years ago I used to use an aluminum pot for cooking rice. I switched to stainless steel.
I also avoid riding in a moving vehicle without wearing a seat belt.
Not crutches, but smart choices for protection from known hazards.
Re: What Is the Safest Cookware to Use?
not the point again but sure ok
active members shape site content. if there is a problem, speak up!
use the report button to flag problematic post content to volunteer moderators' attention.
use the report button to flag problematic post content to volunteer moderators' attention.
Re: What Is the Safest Cookware to Use?
Dietary intakes of some essential and non‐essential trace elements, nitrate, nitrite and N‐nitrosamines, by Dutch adults: Estimated via a 24‐hour duplicate portion study
https://www.tandfonline.com/doi/abs/10. ... 9009373885
"In general the leaching of aluminium is found to contribute only minor amounts to the total dietary intake. With products having a low pH like apple sauce, rhubarb and tomatoes the migration may be considerable, but depends also on the condition of the pan. Reports in the literature that levels of fluoride in foods of about 1 mg/kg might cause strongly enhanced leaching of aluminium leading to contents of up to 150 mg/kg in the food, could not be confirmed by others (Baxter et al. 1988). In our 1984-1985 study 18 out of 110 participants prepared their food in aluminium pans. The mean daily aluminium intake of these 18 persons was 3.2 mg, compared with 3.1 mg for all 110 participants. This result confirms that cooking in aluminium pans does not contribute much to the total dietary intake."
Aluminum Content in Foods and Beverages Consumed in the Spanish Diet
https://onlinelibrary.wiley.com/doi/abs ... .tb15980.x
"The results obtained ranged from 1.362 to 6.610 μg/g in seafood, 0.171 to 29.688 μg/g in vegetables, 19.560 to 70.100 μg/g in olive oil, 0.424 to 6.430 μg/g in dairy products and 25.600 to 58.057 μg/g in stimulant drinks and infusions. This study contributes new data on the Al content of a variety of foods and beverages in Spain and such data are important for composition tables. The higher presence corresponded to seafood, vegetables and dairy products."
Beneficial effect of aluminum on growth of plants adapted to low pH soils
https://www.tandfonline.com/doi/abs/10. ... 7.10414782
Suggested Fertilizer Practices for Blueberries
http://soiltest.uconn.edu/factsheets/Fe ... eberry.pdf
"Aluminum sulfate can also be used to acidify the soil."
Coagulation in Drinking Water Treatment: Particles, Organics and Coagulants
https://iwaponline.com/wst/article-abst ... 11/21/3707
"The paper emphasizes the importance of raw water chemistry, natural organic matter (NOM) concentration and type, and the chemistry of coagulants. ... The removal of NOM with Al coagulants can involve hydrolysis, complexation, precipitation, and adsorption reactions."
Relation between Aluminum Concentrations in Drinking Water and Alzheimer's Disease: An 8-year Follow-up Study
https://academic.oup.com/aje/article/152/1/59/139168
"The relative risk of dementia ... was 1.99 ... for subjects exposed to an aluminum concentration greater than 0.1 mg/liter. This result was confirmed for Alzheimer's disease (adjusted relative risk = 2.14... However, no dose-response relation was found."
edit (found some more):
Effect of dietary aluminum on mineral metabolism of adult males
https://academic.oup.com/ajcn/article-a ... 11/4690900
"During a 40-day balance study, eight adult males were fed two levels of aluminum: 5 mg daily (control diet) and 125 mg daily (test diet). These two levels of dietary aluminum are representative of the upper and lower limits of aluminum that are present in the diets of Americans."
well that could have been better worded, but the point is made nonetheless. back to orig. post:
Human exposure to aluminium
https://pubs.rsc.org/en/content/article ... c3em00374d
"Human activities have circumvented the efficient geochemical cycling of aluminium within the lithosphere and therewith opened a door, which was previously only ajar, onto the biotic cycle to instigate and promote the accumulation of aluminium in biota and especially humans. Neither these relatively recent activities nor the entry of aluminium into the living cycle are showing any signs of abating and it is thus now imperative that we understand as fully as possible how humans are exposed to aluminium and the future consequences of a burgeoning exposure and body burden. The aluminium age is upon us and there is now an urgent need to understand how to live safely and effectively with aluminium."
i have an idea for that.
https://www.tandfonline.com/doi/abs/10. ... 9009373885
"In general the leaching of aluminium is found to contribute only minor amounts to the total dietary intake. With products having a low pH like apple sauce, rhubarb and tomatoes the migration may be considerable, but depends also on the condition of the pan. Reports in the literature that levels of fluoride in foods of about 1 mg/kg might cause strongly enhanced leaching of aluminium leading to contents of up to 150 mg/kg in the food, could not be confirmed by others (Baxter et al. 1988). In our 1984-1985 study 18 out of 110 participants prepared their food in aluminium pans. The mean daily aluminium intake of these 18 persons was 3.2 mg, compared with 3.1 mg for all 110 participants. This result confirms that cooking in aluminium pans does not contribute much to the total dietary intake."
Aluminum Content in Foods and Beverages Consumed in the Spanish Diet
https://onlinelibrary.wiley.com/doi/abs ... .tb15980.x
"The results obtained ranged from 1.362 to 6.610 μg/g in seafood, 0.171 to 29.688 μg/g in vegetables, 19.560 to 70.100 μg/g in olive oil, 0.424 to 6.430 μg/g in dairy products and 25.600 to 58.057 μg/g in stimulant drinks and infusions. This study contributes new data on the Al content of a variety of foods and beverages in Spain and such data are important for composition tables. The higher presence corresponded to seafood, vegetables and dairy products."
Beneficial effect of aluminum on growth of plants adapted to low pH soils
https://www.tandfonline.com/doi/abs/10. ... 7.10414782
Suggested Fertilizer Practices for Blueberries
http://soiltest.uconn.edu/factsheets/Fe ... eberry.pdf
"Aluminum sulfate can also be used to acidify the soil."
Coagulation in Drinking Water Treatment: Particles, Organics and Coagulants
https://iwaponline.com/wst/article-abst ... 11/21/3707
"The paper emphasizes the importance of raw water chemistry, natural organic matter (NOM) concentration and type, and the chemistry of coagulants. ... The removal of NOM with Al coagulants can involve hydrolysis, complexation, precipitation, and adsorption reactions."
Relation between Aluminum Concentrations in Drinking Water and Alzheimer's Disease: An 8-year Follow-up Study
https://academic.oup.com/aje/article/152/1/59/139168
"The relative risk of dementia ... was 1.99 ... for subjects exposed to an aluminum concentration greater than 0.1 mg/liter. This result was confirmed for Alzheimer's disease (adjusted relative risk = 2.14... However, no dose-response relation was found."
edit (found some more):
Effect of dietary aluminum on mineral metabolism of adult males
https://academic.oup.com/ajcn/article-a ... 11/4690900
"During a 40-day balance study, eight adult males were fed two levels of aluminum: 5 mg daily (control diet) and 125 mg daily (test diet). These two levels of dietary aluminum are representative of the upper and lower limits of aluminum that are present in the diets of Americans."
well that could have been better worded, but the point is made nonetheless. back to orig. post:
Human exposure to aluminium
https://pubs.rsc.org/en/content/article ... c3em00374d
"Human activities have circumvented the efficient geochemical cycling of aluminium within the lithosphere and therewith opened a door, which was previously only ajar, onto the biotic cycle to instigate and promote the accumulation of aluminium in biota and especially humans. Neither these relatively recent activities nor the entry of aluminium into the living cycle are showing any signs of abating and it is thus now imperative that we understand as fully as possible how humans are exposed to aluminium and the future consequences of a burgeoning exposure and body burden. The aluminium age is upon us and there is now an urgent need to understand how to live safely and effectively with aluminium."
i have an idea for that.
active members shape site content. if there is a problem, speak up!
use the report button to flag problematic post content to volunteer moderators' attention.
use the report button to flag problematic post content to volunteer moderators' attention.
Re: What Is the Safest Cookware to Use?
"I have an idea for that."
please elaborate...
please elaborate...
Re: What Is the Safest Cookware to Use?
certainly. per the above http://www.thisisms.com/forum/general-d ... ml#p254122
active members shape site content. if there is a problem, speak up!
use the report button to flag problematic post content to volunteer moderators' attention.
use the report button to flag problematic post content to volunteer moderators' attention.

