How EBV causes MS
Posted: Tue Apr 16, 2019 2:11 am
In this review, the authors propose an integrated model of EBV involvement in MS pathology
Epstein-Barr Virus in Multiple Sclerosis
https://www.intechopen.com/online-first ... -sclerosis
Some excerpts:
Whatever the initial cellular target, one thing is fairly well-established; the cellular site of long-term EBV persistence is B-lymphocytes. These cells can be transformed and immortalized by EBV when grown in in vitro cultures.
[...] an efficiently functioning immune system is essential to keep EBV infection under control and maintain a homeostatic virus-host relationship. Thus, any disruption of the intricate connection between EBV and the immune system can lead to serious health conditions, for instance EBV-induced malignancies and some autoimmune disorders such as MS.
[...] CD8+ T cells in the blood of MS patients with inactive disease, have been shown to express the immune inhibitory molecule, programmed death 1 (PD-1), making these cells less efficient in eliminating EBV infected cells [...]
Surprisingly, we found EBV not only in B-cells, but also in astrocytes and some microglial cells. Significantly, the virus was transcriptionally active in these cells [...]
[...] the identity of the target antigen for the autoreactive T cells remains elusive. A very recent study has reported that intrathecal CD4+ T cells from HLA-DRB3 positive MS patients reacted with GDP-L-fucose synthase, an enzyme frequently expressed in human cells as well as in bacteria commonly present in the gastrointestinal track of MS patients.
We propose that EBV infected memory B-cells act as antigen presenting cells (APC), resulting in the activation of helper T-cells, which in individuals carrying certain HLA haplotypes, activate autoreactive B and T-cells targeting antigens expressed on oligodendrocytes (Figure 1)
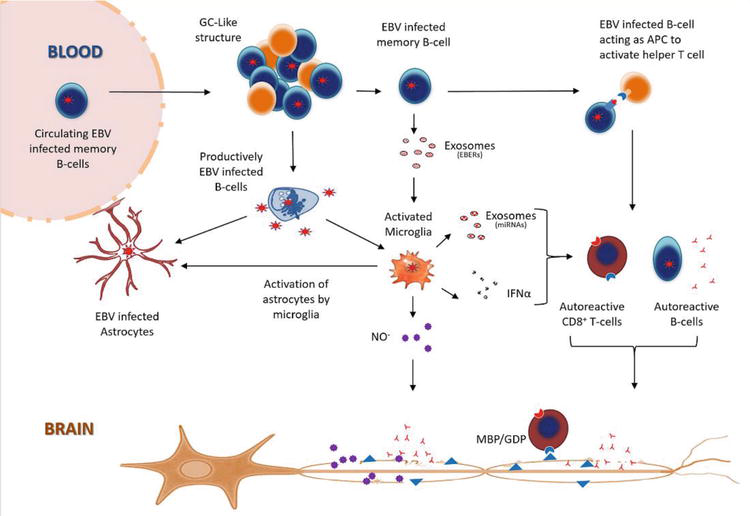
Since astrocytes interact with blood vessels to form the BBB, any functional impact on these cells could also increase BBB permeability and exacerbate infiltration of peripheral immune cells into the CNS. This could explain the characteristic perivascular cuffing and presence of inflammatory aggregates resembling germinal center (GC)-like structures commonly observed in the CNS in viral infections
Epstein-Barr Virus in Multiple Sclerosis
https://www.intechopen.com/online-first ... -sclerosis
Some excerpts:
Whatever the initial cellular target, one thing is fairly well-established; the cellular site of long-term EBV persistence is B-lymphocytes. These cells can be transformed and immortalized by EBV when grown in in vitro cultures.
[...] an efficiently functioning immune system is essential to keep EBV infection under control and maintain a homeostatic virus-host relationship. Thus, any disruption of the intricate connection between EBV and the immune system can lead to serious health conditions, for instance EBV-induced malignancies and some autoimmune disorders such as MS.
[...] CD8+ T cells in the blood of MS patients with inactive disease, have been shown to express the immune inhibitory molecule, programmed death 1 (PD-1), making these cells less efficient in eliminating EBV infected cells [...]
Surprisingly, we found EBV not only in B-cells, but also in astrocytes and some microglial cells. Significantly, the virus was transcriptionally active in these cells [...]
[...] the identity of the target antigen for the autoreactive T cells remains elusive. A very recent study has reported that intrathecal CD4+ T cells from HLA-DRB3 positive MS patients reacted with GDP-L-fucose synthase, an enzyme frequently expressed in human cells as well as in bacteria commonly present in the gastrointestinal track of MS patients.
We propose that EBV infected memory B-cells act as antigen presenting cells (APC), resulting in the activation of helper T-cells, which in individuals carrying certain HLA haplotypes, activate autoreactive B and T-cells targeting antigens expressed on oligodendrocytes (Figure 1)

Since astrocytes interact with blood vessels to form the BBB, any functional impact on these cells could also increase BBB permeability and exacerbate infiltration of peripheral immune cells into the CNS. This could explain the characteristic perivascular cuffing and presence of inflammatory aggregates resembling germinal center (GC)-like structures commonly observed in the CNS in viral infections