Fatigue reduced by a single dose of vitamin D (100,000 IU) – RCT Dec 2016
Effect of vitamin D3 on self-perceived fatigue: A double-blind randomized placebo-controlled trial.
Medicine (Baltimore). 2016 Dec;95(52):e5353. doi: 10.1097/MD.0000000000005353.
Nowak A1, Boesch L, Andres E, Battegay E, Hornemann T, Schmid C, Bischoff-Ferrari HA, Suter PM, Krayenbuehl PA.
aDepartment of Internal Medicine
bInstitute for Clinical Chemistry, University Hospital Zurich and University of Zurich
cDivision of Endocrinology, Diabetology and Clinical Nutrition
dDepartment of Geriatrics, University Hospital Zurich, Zurich
eDepartment of Internal Medicine, Spital Linth, Uznach, Switzerland.
BACKGROUND:
Vitamin D deficiency is frequent and has been associated with fatigue in uncontrolled trials.
METHODS:
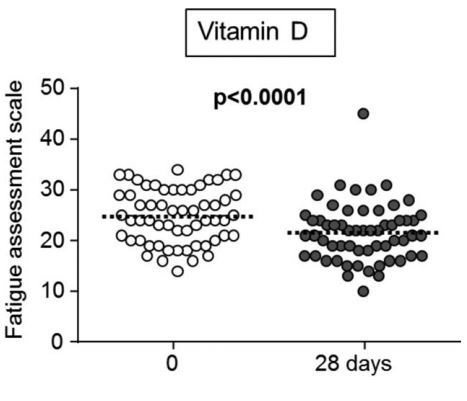
This is the first double-blind placebo-controlled clinical trial to investigate the efficacy of per os vitamin D3 (cholecalciferol) in treating fatigue among otherwise healthy persons with low serum 25-hydroxyvitamin D (25(OH)D) levels. We enrolled 120 individuals (mean age 29 ± 6 years, 53% women) presenting with fatigue and vitamin D deficiency (serum 25(OH)D < 20 µg/L). Participants were randomized to a single oral dose of 100,000 units of vitamin D or placebo. The primary endpoint was intra-individual change in the fatigue assessment scale (FAS) at 4 weeks after treatment.
RESULT:
The mean age of the participants was 29 ± 6 years, 53% were women. Mean FAS decreased significantly more in the vitamin D group (-3.3 ± 5.3; 95% confidence interval [CI] for change -14.1 to 4.1) compared with placebo (-0.8 ± 5.3; 95% CI for change -9.0 to 8.7); (P = 0.01). Amelioration of fatigue was reported more frequently in vitamin D than in placebo group (42 [72%] vs. 31 [50%]; P = 0.01; odds ratio [OR] 2.63, 95% CI for OR 1.23-5.62). Among all participants, improvement in fatigue score correlated with the rise in 25(OH)D level (R = -0.22, P = 0.02).
CONCLUSION:
Vitamin D treatment significantly improved fatigue in otherwise healthy persons with vitamin D deficiency.
This study was registered at the
http://www.ClinicalTrials.gov Protocol ID NCT02022475.
PMID: 28033244 DOI: 10.1097/MD.0000000000005353