2 Years Post HSCT And MS Still Stopped
Re: 2 Years Post HSCT And MS Still Stopped
Lisa is one of the folks who started me on the journey to try to get this treatment (along of course with You guys around here) and she is a great lady. I wish her the best.
"A gun is a tool, Marian; no better or no worse than any other tool: an axe, a shovel or anything. A gun is as good or as bad as the man using it. Remember that." -- Shane
Who is John Galt?
Who is John Galt?
- georgegoss
- Family Elder
- Posts: 284
- Joined: Sat Oct 30, 2010 2:00 pm
- Location: California
- Contact:
Re: 2 Years Post HSCT And MS Still Stopped
Indeed, Lisa is a wonderful person. I'm confident her cyclophosphamide retreatments will effectively, and lastingly knock her MS into full remission and stop all further underlying MS disease activity. No doubt she deserves as much.
- Chilax
- Getting to Know You...
- Posts: 24
- Joined: Sat Dec 04, 2010 3:00 pm
- Location: New Jersey (USA)
- Contact:
Re: 2 Years Post HSCT And MS Still Stopped
Lisa is a tremendous person who, along with her husband Jim, helped me tremendously in successfully having my HSCT on 8/22/11 at NW. She will overcome this temporary speed bump with the six monthly treatments ordered by Dr. Burt and her MS will soon be a distant memory. She is a very strong and decent person with a very supportive family. In fact she and Jim visited me at NW last August while I was recovering nicely after the stem cell transplant.
Al
Al
Re: 2 Years Post HSCT And MS Still Stopped
I think in SPMS and PPMS, MRI activity is not as obvious. This could be due to a switch from inflamation which shows up on MRI to neurodegeneration which is gray matter damage that is harder to detect.KateCW wrote:My doctors describe my lesions as vey subtle, yet I am an EDSS of 8. The nature of PPMS I guess.
Here is a good graphic of this. You can see MRI activity (arrows) decreases once you transition to SPMS:
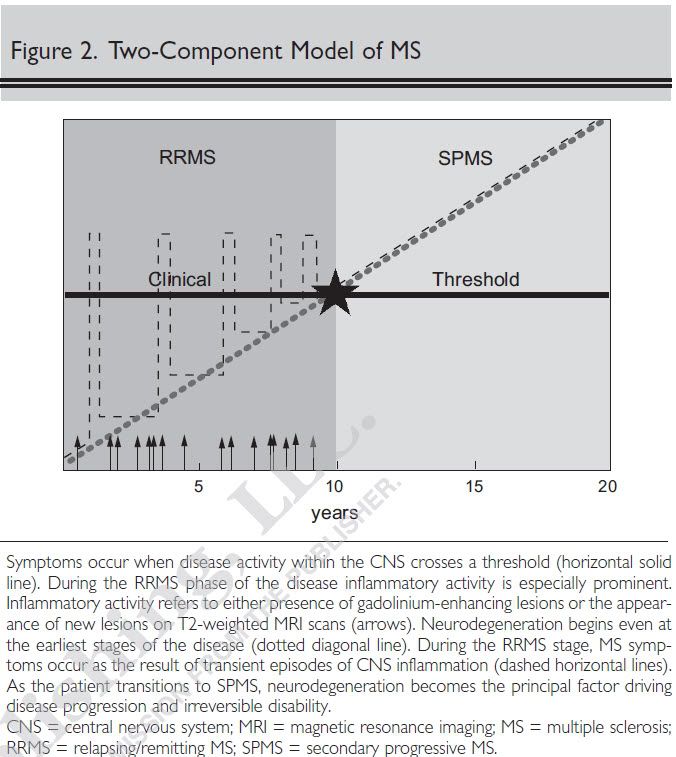
- georgegoss
- Family Elder
- Posts: 284
- Joined: Sat Oct 30, 2010 2:00 pm
- Location: California
- Contact:
Re: 2 Years Post HSCT And MS Still Stopped
A wonderful graph, CV. And a very accurate description of the most common presentation of MS evolution (same as my own).
- georgegoss
- Family Elder
- Posts: 284
- Joined: Sat Oct 30, 2010 2:00 pm
- Location: California
- Contact:
Re: 2 Years Post HSCT And MS Still Stopped
I hope no one will take this as me bashing Burt's non-myeloablative HSCT protocol. I'm not. I totally support Dr. Burt's work and his non-myeloablative HSCT protocol. But I think highlighting the facts of treatment results important for understanding the nuances in the differences between the myeloablative and non-myeloablative protocols. The following is what I have previously communicated to other people on the Facebook "HSCT for MS" forum. . . . .
This "new lesion" activity following Burt's non-myeloablative HSCT protocol, although unfortunate, is quite interesting to me. A retrospective analysis of the phase II study results indicates that fully 20% of HSCT-treated patients in Burt's non-myeloablative program do not experience a 100% stopping of the underlying MS disease activity following the treatment. That seems to me like quite a large number. However, all of these 1-in-5 (RRMS) patients have been able to knock the MS back into complete remission following a retreatment regimen incorporating various immuno-ablative agents that further reduce the the population of remaining in-vivo autoreactive lymphocytes; now mainly six monthly cyclophosphamide infusions. It seems logical that this might occur with the more 'gentle' non-myeloablative protocol that has not been shown to occur with the fully myeloabltive (BEAM) HSCT protocol that has seen no post-treatment relapses. It all comes down to this:
http://2.bp.blogspot.com/-3w43aw2GsWA/T ... rofile.jpg
From the technical literature of Burt's non-myeloablative phase II clinical trial. . . .
Five patients (23%) relapsed at an average of 11 months after transplantation after an initial improvement in neurological function. Patients who relapsed were retreated with immunosuppressants: one was treated with daclizumab, one with mycophenolate mofetil, two with intravenous cyclophosphamide for 6 months, and one with intravenous cyclophosphamide for 6 months followed by maintenance interferon beta. The patients have all achieved further remissions with no further relapses and continued improvement in EDSS score.
I'm really wondering if Dr. Burt doesn't want to treat progressive MS patients (even though they can benefit from HSCT) because if his protocol "fails" to render a curative result (stopping of underlying disease activity), then how would they know this? Quite difficult to identify in progressive cases, especially if it is expected that 20% of treated patients would not experience disease remission following initial treatment. This is why I favor the myeloablative HSCT protocol for all progressive (PPMS, SPMS) cases that virtually ensures complete remission, and either of the two protocols for relapsing cases. That way if the non-myeloablative protocol fails in a relapsing patient then it will be easy to identify and get them on a post-HSCT retreatment regimen.
This "new lesion" activity following Burt's non-myeloablative HSCT protocol, although unfortunate, is quite interesting to me. A retrospective analysis of the phase II study results indicates that fully 20% of HSCT-treated patients in Burt's non-myeloablative program do not experience a 100% stopping of the underlying MS disease activity following the treatment. That seems to me like quite a large number. However, all of these 1-in-5 (RRMS) patients have been able to knock the MS back into complete remission following a retreatment regimen incorporating various immuno-ablative agents that further reduce the the population of remaining in-vivo autoreactive lymphocytes; now mainly six monthly cyclophosphamide infusions. It seems logical that this might occur with the more 'gentle' non-myeloablative protocol that has not been shown to occur with the fully myeloabltive (BEAM) HSCT protocol that has seen no post-treatment relapses. It all comes down to this:
http://2.bp.blogspot.com/-3w43aw2GsWA/T ... rofile.jpg
From the technical literature of Burt's non-myeloablative phase II clinical trial. . . .
Five patients (23%) relapsed at an average of 11 months after transplantation after an initial improvement in neurological function. Patients who relapsed were retreated with immunosuppressants: one was treated with daclizumab, one with mycophenolate mofetil, two with intravenous cyclophosphamide for 6 months, and one with intravenous cyclophosphamide for 6 months followed by maintenance interferon beta. The patients have all achieved further remissions with no further relapses and continued improvement in EDSS score.
I'm really wondering if Dr. Burt doesn't want to treat progressive MS patients (even though they can benefit from HSCT) because if his protocol "fails" to render a curative result (stopping of underlying disease activity), then how would they know this? Quite difficult to identify in progressive cases, especially if it is expected that 20% of treated patients would not experience disease remission following initial treatment. This is why I favor the myeloablative HSCT protocol for all progressive (PPMS, SPMS) cases that virtually ensures complete remission, and either of the two protocols for relapsing cases. That way if the non-myeloablative protocol fails in a relapsing patient then it will be easy to identify and get them on a post-HSCT retreatment regimen.
Re: 2 Years Post HSCT And MS Still Stopped
as i proceed down my 'road' this is certainly something i am thinking about. granted, i am currently rrms, but the thought of going thru hsct and then having to "possibly" go thru more treatment afterwards makes me think that the myeloblative is the best option. an issue of course is finding a reputable hospital in which you qualify for the procedure.
the day this becomes FDA approved will be wonderful. patience.
the day this becomes FDA approved will be wonderful. patience.
Re: 2 Years Post HSCT And MS Still Stopped
On that note I have heard it said that HSCT trials are in the phase three stage. Which trial is that reffering to?
Also, what phase of trial is the procedure by Dr. Burt in?
Also, what phase of trial is the procedure by Dr. Burt in?
- georgegoss
- Family Elder
- Posts: 284
- Joined: Sat Oct 30, 2010 2:00 pm
- Location: California
- Contact:
Re: 2 Years Post HSCT And MS Still Stopped
Burt's non-myeloablative MIST trial is currently in Phase III and treatment of this larger randomized patient population is currently underway:
http://clinicaltrials.gov/ct2/show/NCT0 ... sis&rank=2
The fully myeloblative (BEAM) study run out of Fred Hutchinson in Seattle has already completed treatment of all phase II trial patients and is currently performing the follow-up monitoring of the patients that will be completed over the next several years. However, the phase III trial plans have already been submitted to the FDA for approval and the phase III patient treatment will begin likely sometime in 2014 (hopefully a little earlier if possible). The following is just the phase II description. The phase III description should be the same, except with a larger patient treatment population and randomization:
http://clinicaltrials.gov/ct2/show/NCT0 ... tle&rank=1
BTW Shaight. . . Like yourself I also was thinking about these issues at the time I was making the decision to go for HSCT for my own MS in 2009. Unfortunately there wasn't as much available data at that time as there is today. With what is known now I still favor the myeloablative protocol "in general" for progressive cases. However, if one does not have the option available, as a progressive patient I still would jump at the chance to have the non-myeloablative protocol because it still has a far higher probability & efficacy compared to any other treatment currently available. However, since you are still RRMS and the transition from RR-to-SP doesn't happen overnight, I think you're likely going to be good with the non-myeloablative protocol. But even if you do fall into the the roughly 20-25% of patients that relapse (or show lesion activity) following treatment, it should be easy to identify and then take six monthly IV infusions of cyclophosphamide that will be the finishing touch to the treatment. And of course you have a four-out-of-five chance the treatment will get it the first time.
http://clinicaltrials.gov/ct2/show/NCT0 ... sis&rank=2
The fully myeloblative (BEAM) study run out of Fred Hutchinson in Seattle has already completed treatment of all phase II trial patients and is currently performing the follow-up monitoring of the patients that will be completed over the next several years. However, the phase III trial plans have already been submitted to the FDA for approval and the phase III patient treatment will begin likely sometime in 2014 (hopefully a little earlier if possible). The following is just the phase II description. The phase III description should be the same, except with a larger patient treatment population and randomization:
http://clinicaltrials.gov/ct2/show/NCT0 ... tle&rank=1
BTW Shaight. . . Like yourself I also was thinking about these issues at the time I was making the decision to go for HSCT for my own MS in 2009. Unfortunately there wasn't as much available data at that time as there is today. With what is known now I still favor the myeloablative protocol "in general" for progressive cases. However, if one does not have the option available, as a progressive patient I still would jump at the chance to have the non-myeloablative protocol because it still has a far higher probability & efficacy compared to any other treatment currently available. However, since you are still RRMS and the transition from RR-to-SP doesn't happen overnight, I think you're likely going to be good with the non-myeloablative protocol. But even if you do fall into the the roughly 20-25% of patients that relapse (or show lesion activity) following treatment, it should be easy to identify and then take six monthly IV infusions of cyclophosphamide that will be the finishing touch to the treatment. And of course you have a four-out-of-five chance the treatment will get it the first time.
Re: 2 Years Post HSCT And MS Still Stopped
Hi Shucks, I was just wondering, because we are in the process to traveling to Chicago to see Dr Burt for an evaluation....what was his explanation for the incentive that the people who are not randomized to the transplant arm have to not drop from the tial. Thanks.
Re: 2 Years Post HSCT And MS Still Stopped
This study was only open to Relapsing Remitting and Progressive Relapsing MS. They must know something about MS that we don't.The fully myeloblative (BEAM) study run out of
Fred Hutchinson in Seattle has already completed
treatment of all phase II trial patients and is
currently performing the follow-up monitoring of
the patients that will be completed over the next
several years.
- georgegoss
- Family Elder
- Posts: 284
- Joined: Sat Oct 30, 2010 2:00 pm
- Location: California
- Contact:
Re: 2 Years Post HSCT And MS Still Stopped
It comes back again to HSCT working "best" on relapsing patients. However, because HSCT is also effective in all forms of MS (including progressive cases, albeit less dramatically) I "imagine" they included RP cases so they can easily see if there are any cases that relapse (there haven't been any). Other than this guess, someone would have to discover the real reason directly from the principal researches that defined the inclusion/exclusion criteria.CVfactor wrote:This [HALT-MS] study was only open to Relapsing Remitting and Progressive Relapsing MS. They must know something about MS that we don't.
Re: 2 Years Post HSCT And MS Still Stopped
It looks like the trial run by Dr. Burt does not really distinguish between the types of MS but only requires an EDSS score between 2 and 6.
I would imagine he would have some people in the trial that are PPMS, SPMS and PRMS with EDSS scores near 6.
So far there are only problems with a small portion who relapse that require additional chemotherapy, but is there any indication of how the people with an initially high EDSS score are doing? Has progression stopped?
I would imagine he would have some people in the trial that are PPMS, SPMS and PRMS with EDSS scores near 6.
So far there are only problems with a small portion who relapse that require additional chemotherapy, but is there any indication of how the people with an initially high EDSS score are doing? Has progression stopped?
- Chilax
- Getting to Know You...
- Posts: 24
- Joined: Sat Dec 04, 2010 3:00 pm
- Location: New Jersey (USA)
- Contact:
Re: 2 Years Post HSCT And MS Still Stopped
CVfactor-
In addition to an EDSS score between 2.0 and 6.0 and a diagnosis of RRMS ('Inclusion' criteria), Dr. Burt's 'Exclusion' criteria include:
14.Diagnosis of primary progressive MS.
15.Diagnosis of secondary progressive MS
That said, I do know of one other person that had their HSCT with me last year (8/22/11). Pre-HSCT he was not ambulatory or barely ambulatory indicative of EDSS score of around 6. He has left his wheelchair behind and only partially uses a walker and is now using mostly his cane. Hopefully the cane will also be a thing of the past for him as well!
Further details on Dr. Burt's Phase III clinical trial are contained within the attached link.
http://clinicaltrials.gov/ct2/show/NCT0 ... sis&rank=2
Al
In addition to an EDSS score between 2.0 and 6.0 and a diagnosis of RRMS ('Inclusion' criteria), Dr. Burt's 'Exclusion' criteria include:
14.Diagnosis of primary progressive MS.
15.Diagnosis of secondary progressive MS
That said, I do know of one other person that had their HSCT with me last year (8/22/11). Pre-HSCT he was not ambulatory or barely ambulatory indicative of EDSS score of around 6. He has left his wheelchair behind and only partially uses a walker and is now using mostly his cane. Hopefully the cane will also be a thing of the past for him as well!
Further details on Dr. Burt's Phase III clinical trial are contained within the attached link.
http://clinicaltrials.gov/ct2/show/NCT0 ... sis&rank=2
Al
Re: 2 Years Post HSCT And MS Still Stopped
Thanks Chilax,
Glad to see everything is going good after the treatment.
I should have read further down, but it does indeed exclude PPMS and SPMS. Did Dr. Burt give any reason why these types of MS are excluded from the trial?
Glad to see everything is going good after the treatment.
I should have read further down, but it does indeed exclude PPMS and SPMS. Did Dr. Burt give any reason why these types of MS are excluded from the trial?