The NERVGEN-291 miracle!
Re: The NERVGEN-291 miracle!
Here's a recent presentation from NervGen. If the clinical data is as good as their preclinical videos in the presentation, then this could be a game changer.
https://nervgen.com/wp-content/uploads/ ... 01-web.pdf
https://nervgen.com/wp-content/uploads/ ... 01-web.pdf
Re: The NERVGEN-291 miracle!
Hope so...
Re: The NERVGEN-291 miracle!
Here's an interview with the two founders of NervGen and the CEO. They discuss the background science leading to the development of NVG-291, the design of the current phase 1b-2a trial and plans for a larger phase 2 trial.
Re: The NERVGEN-291 miracle!
I think we have to remember that (if this works) we could possibly remyelinate damaged pathways. It doesn't imply that the causative agent is eliminated. That's a problem that still needs to be solved. If the causative agent turned out to be EBV, then you would still need to treat that.
Nonetheless, it's a possibly a big step forward.
Nonetheless, it's a possibly a big step forward.
Re: The NERVGEN-291 miracle!
This paper from 2018 focused on MS (EAE).
Modulation of proteoglycan receptor PTPσ enhances MMP-2 activity to promote recovery from multiple sclerosis
Nature Commun. 2018 Oct 8;9(1):4126.
Multiple Sclerosis (MS) is characterized by focal CNS inflammation leading to the death of oligodendrocytes (OLs) with subsequent demyelination, neuronal degeneration, and severe functional deficits. Inhibitory chondroitin sulfate proteoglycans (CSPGs) are increased in the extracellular matrix in the vicinity of MS lesions and are thought to play a critical role in myelin regeneration failure. We here show that CSPGs curtail remyelination through binding with their cognate receptor, protein tyrosine phosphatase σ (PTPσ) on oligodendrocyte progenitor cells (OPCs). We report that inhibition of CSPG/PTPσ signaling by systemically deliverable Intracellular Sigma Peptide (ISP), promotes OPC migration, maturation, remyelination, and functional recovery in animal models of MS. Furthermore, we report a downstream molecular target of PTPσ modulation in OPCs involving upregulation of the protease MMP-2 that allows OPCs to enzymatically digest their way through CSPGs. In total, we demonstrate a critical role of PTPσ/CSPG interactions in OPC remyelination in MS.
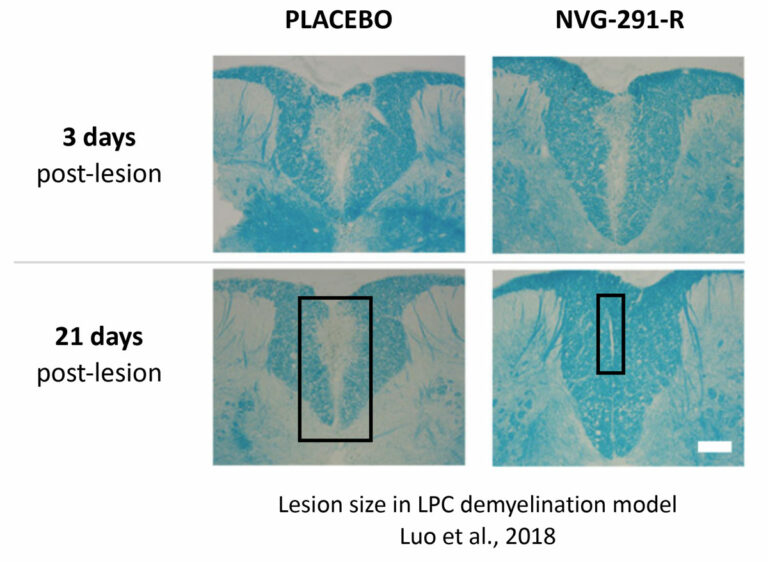
Free full text
Modulation of proteoglycan receptor PTPσ enhances MMP-2 activity to promote recovery from multiple sclerosis
Nature Commun. 2018 Oct 8;9(1):4126.
Multiple Sclerosis (MS) is characterized by focal CNS inflammation leading to the death of oligodendrocytes (OLs) with subsequent demyelination, neuronal degeneration, and severe functional deficits. Inhibitory chondroitin sulfate proteoglycans (CSPGs) are increased in the extracellular matrix in the vicinity of MS lesions and are thought to play a critical role in myelin regeneration failure. We here show that CSPGs curtail remyelination through binding with their cognate receptor, protein tyrosine phosphatase σ (PTPσ) on oligodendrocyte progenitor cells (OPCs). We report that inhibition of CSPG/PTPσ signaling by systemically deliverable Intracellular Sigma Peptide (ISP), promotes OPC migration, maturation, remyelination, and functional recovery in animal models of MS. Furthermore, we report a downstream molecular target of PTPσ modulation in OPCs involving upregulation of the protease MMP-2 that allows OPCs to enzymatically digest their way through CSPGs. In total, we demonstrate a critical role of PTPσ/CSPG interactions in OPC remyelination in MS.

Free full text
Re: The NERVGEN-291 miracle!
Very promising.
Pain! You made me a, you made me a believer, believer
Pain! You break me down, you build me up, believer, believer
Pain! Oh let the bullets fly, oh let them rain
My life, my love, my drive, it came from... Pain!
Pain! You break me down, you build me up, believer, believer
Pain! Oh let the bullets fly, oh let them rain
My life, my love, my drive, it came from... Pain!
Re: The NERVGEN-291 miracle!
NervGen Pharma on Track to Complete Enrollment, Deliver Data Readout in Phase 1b/2a Clinical Trial for NVG-291 in Spinal Cord Injury
https://www.newsfilecorp.com/release/19 ... ord-Injury
https://www.newsfilecorp.com/release/19 ... ord-Injury
- On track to complete enrollment of the chronic cohort in Q2 2024
- Data readout from chronic cohort expected in Q3 2024
- Planning underway to make NVG-291 available to placebo-treated subjects following cohort completion
Re: The NERVGEN-291 miracle!
Thanks for the article. I agree with the author that it's likely a good sign that NervGen will be offering NVG-291 to patients on the placebo arm in an open label extension. They probably wouldn't even consider it if NVG-291 turned out to be ineffective.
However, the following statement appears to contradict the available data. The author wrote...
"In recent weeks, the company’s share price has surged higher a couple of times in response to hints that NervGen’s ongoing proof-of-concept Phase 1b/2a clinical trial in humans may actually be working."
Almost two months ago the share price was $2.82 on Feb 7th. It's now $1.52 as of closing on Mar 26th. However, going back a little further it was $1.17 on Dec 12th. Thus, there has been an increase, but the shares have been coming back down for the last 4-5 weeks.
https://www.barrons.com/market-data/stocks/ngenf
Re: The NERVGEN-291 miracle!
NervGen Pharma Nears Completion of NVG-291 Spinal Cord Injury Trial Enrollment and Data Release
May 27, 2024
https://synapse-patsnap-com.libproxy1.n ... ta-release
NervGen Pharma Corp., a biotech firm focused on nervous system repair, is nearing the completion of participant recruitment for its Phase 1b/2a clinical trial of NVG-291, a potential spinal cord injury treatment. The company anticipates releasing data from the chronic cohort in the third quarter of 2024. NVG-291 has shown promise in preclinical studies for both acute and chronic spinal cord injuries. The trial, which is taking place at the Shirley Ryan AbilityLab in Chicago, a renowned center for severe and complex medical conditions, is designed to assess the drug's efficacy using a variety of measures, including clinical outcomes and electrophysiological assessments.
NervGen is also planning a subsequent study where participants who were initially given a placebo will have the opportunity to receive NVG-291 under an open-label protocol, pending positive efficacy signals and regulatory approvals. The company's Chief Medical Officer, Dan Mikol, expressed optimism about the study's progress and the potential for NVG-291 to become the first approved treatment for spinal cord injuries.
The Shirley Ryan AbilityLab team, led by Monica A. Perez, is enthusiastic about the ongoing trial and the innovative design incorporating electrophysiology. The trial is partially funded by a grant from Wings for Life, an organization supporting the translation of scientific discoveries into therapeutic applications.
NVG-291 is a novel therapeutic peptide derived from the receptor protein tyrosine phosphatase sigma (PTPσ) and has received Fast Track Designation from the FDA. NervGen is committed to developing treatments that promote self-repair of the nervous system following injury or disease, with spinal cord injury as its primary focus.
May 27, 2024
https://synapse-patsnap-com.libproxy1.n ... ta-release
NervGen Pharma Corp., a biotech firm focused on nervous system repair, is nearing the completion of participant recruitment for its Phase 1b/2a clinical trial of NVG-291, a potential spinal cord injury treatment. The company anticipates releasing data from the chronic cohort in the third quarter of 2024. NVG-291 has shown promise in preclinical studies for both acute and chronic spinal cord injuries. The trial, which is taking place at the Shirley Ryan AbilityLab in Chicago, a renowned center for severe and complex medical conditions, is designed to assess the drug's efficacy using a variety of measures, including clinical outcomes and electrophysiological assessments.
NervGen is also planning a subsequent study where participants who were initially given a placebo will have the opportunity to receive NVG-291 under an open-label protocol, pending positive efficacy signals and regulatory approvals. The company's Chief Medical Officer, Dan Mikol, expressed optimism about the study's progress and the potential for NVG-291 to become the first approved treatment for spinal cord injuries.
The Shirley Ryan AbilityLab team, led by Monica A. Perez, is enthusiastic about the ongoing trial and the innovative design incorporating electrophysiology. The trial is partially funded by a grant from Wings for Life, an organization supporting the translation of scientific discoveries into therapeutic applications.
NVG-291 is a novel therapeutic peptide derived from the receptor protein tyrosine phosphatase sigma (PTPσ) and has received Fast Track Designation from the FDA. NervGen is committed to developing treatments that promote self-repair of the nervous system following injury or disease, with spinal cord injury as its primary focus.
Re: The NERVGEN-291 miracle!
NVG-291 shows improvement in a stroke model.
Re: The NERVGEN-291 miracle!
In this presentation, Nervgen anticipates wide applicability of NVG-291 to diseases such as spinal cord injury, Multiple Sclerosis, Alzheimer's, stroke, traumatic brain injury and others. Nervgen also anticipates that treatment, once approved, could cost in the range of $200,000.
https://www.youtube.com/watch?v=ds3ePg_-TbE
https://www.youtube.com/watch?v=ds3ePg_-TbE
Re: The NERVGEN-291 miracle!
With 200.000$ per year is a rich people only treatment!
It isn't a lifetime therapy, just until someone achieves mobility then will stop it?
It isn't a lifetime therapy, just until someone achieves mobility then will stop it?
