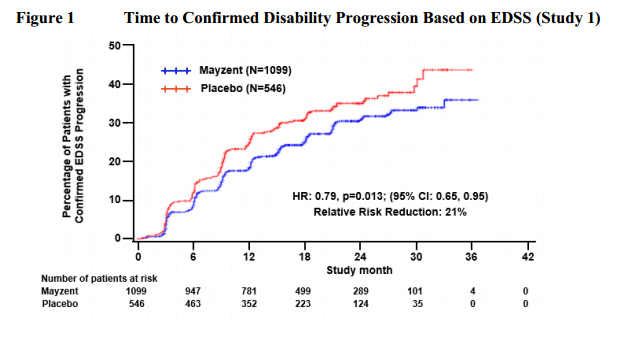
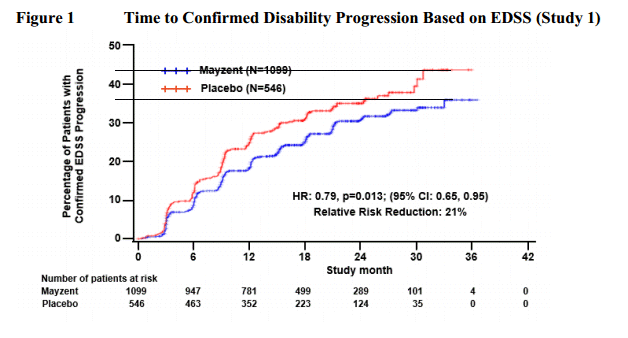
Hi all,dignan wrote:Upon request, here are the drugs from the pipeline that are being tested for progressive forms of MS. If I've missed anything, please let me know.
Progressive MS Treatment Pipeline - September 2011
Phase 3 Trials
- Gilenya (aka FTY720 and fingolimod) (PPMS) (Novartis)
- Masitinib (SPMS and PPMS) (aka Kinavet or AB1010) (AB Science)
- Ocrelizumab (R1594) (trial for PPMS) (Genentech, Roche, Biogen)
- Revimmune (aka high dose cyclophosphamide or cytoxan) (Accentia Biopharmaceuticals) (refractory MS - includes SPMS I think, not sure about PPMS)
Phase 2 Trials
- Amiloride (Oxford University) (PPMS)
- Fluoxetine (aka Prozac) (SP, PP & RRMS) (University Medical Center Groningen)
- GEM-SP (SPMS) (Gemacbio)
- Hydroxyurea (PPMS) (S. Andrea Hospital)
- Idebenone (National Institute of Neurological Disorders and Stroke)
- Lithium (US Department of Veterans Affairs)
- Mesenchymal Stem Cells (4 trials listed in clinicaltrials.gov)
- MIS416 (progressive MS) (Innate Therapeutics)
- PI-2301 (Peptimmune)
- Pixantrone (aka BBR 2778) (SPMS) (Cell Therapeutics)
- RTL1000 (Artielle ImmunoTherapeutics)
- Zocor (aka simvastatin) (SPMS) (MS-STAT trial - UK)
Can anyone pls update this status?
I tried to find something on the net but didn't get anything...
Thks


 . They have run a previous trial using autologous cells, and previous to that, a single original trial patient. From my understanding the original single guy improved, the second trial was supposedly successful based on a company bought up the rights, and is now running an allogeneic based trial. (see link, in the link) I reiterate, this trial has already started dosing patients. I tried to get onto the second trial, but although the dr was in Australia, he only included patients from his hospital
. They have run a previous trial using autologous cells, and previous to that, a single original trial patient. From my understanding the original single guy improved, the second trial was supposedly successful based on a company bought up the rights, and is now running an allogeneic based trial. (see link, in the link) I reiterate, this trial has already started dosing patients. I tried to get onto the second trial, but although the dr was in Australia, he only included patients from his hospital